Empirical vs. Molecular Formula: The Fundamental Clash in Chemical Representation
Empirical vs. Molecular Formula: The Fundamental Clash in Chemical Representation
Standing at the crossroads of chemistry’s evolution, the tension between empirical formulas and molecular formulas reveals more than just two notational styles—it exposes a foundational shift in how scientists understand matter. Empirical formulas depict the simplest whole-number ratio of atoms in a compound, reducing complexity to its most basic expression, while molecular formulas reveal both the elemental composition and the actual atomic arrangement—down to discrete molecules. This distinction, though subtle in appearance, profoundly impacts analytical chemistry, pharmaceutical development, and material science.
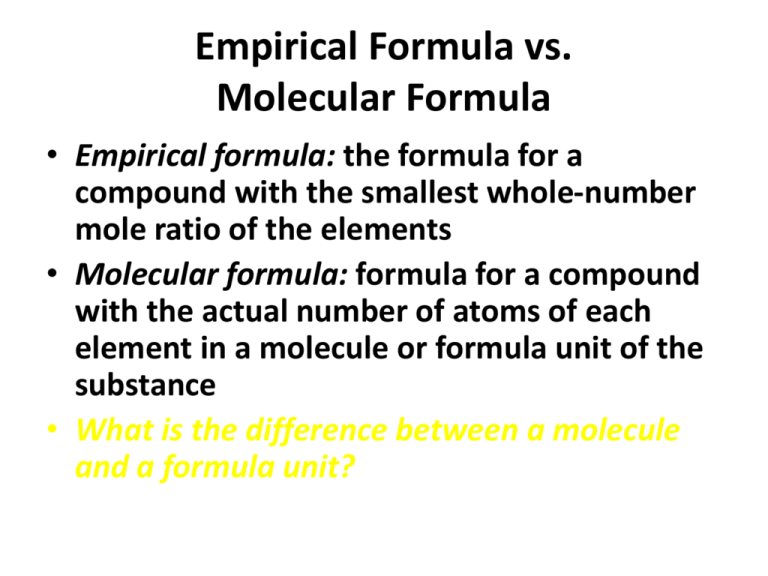
Empirical formulas offer a stripped-down truth. Defined as the lowest integer ratio of atoms derived from mass analysis, they speak to stoichiometry’s pragmatic core: “What is the simplest whole-number composition of this compound?” For example, glucose (C₆H₁₂O₆), with its complex molecular structure, reduces to the empirical formula CH₂O—a clear indicator of carbon, hydrogen, and oxygen in a 1:2:1 ratio. Empirical formulas are indispensable in chemical reactions involving exact mass balances, where inductive reasoning about molecular weight or structure cannot suffice.
The Power of Empirical Precision in Analysis
Empirical formulas shine in quantitative contexts such as combustion analysis, gravimetry, and titrations. They allow chemists to verify reaction stoichiometry without guessing molecular architecture, focusing instead on atomic equivalence. As noted by chemist Gary G.Miessler: “Empirical formulas strip away complexity to focus on relative proportions—essential when exact structure remains unknown.” This functionality makes them indispensable in industrial quality control, where maintaining precise stoichiometric ratios ensures product consistency and safety.
While empirical formulas resolve "how many atoms by ratio", molecular formulas answer "what atoms exist and in what order." Molecular formulas reflect not just atomic proportions but also the actual bonding configuration, crucial for understanding molecular properties, reactivity, and function. For instance, in glucose (C₆H₁₂O₆), the molecular formula reveals alternating carbon-carbon bonds, hydroxyl groups, and an open-chain structure—information essential for biochemical interactions.
Unlike empirical formulas, molecular formulas expose the molecular architecture behind chemical behavior.
Decoding Complexity: From Empirical to Molecular
Transitioning from empirical to molecular representation involves integrating molecular weight data, typically obtained through mass spectrometry or infrared spectroscopy. The empirical formula provides the backbone ratio, while molecular formulae add SVOH detail—hydrogen, carbon, oxygen, and sometimes nitrogen or halides—using precise atomic mass values.This layered approach aligns empirical simplicity with molecular depth, enabling scientists to bridge qualitative and quantitative chemistry.
Such integration is vital in drug discovery: knowing a molecule’s molecular formula confirms its pharmacological identity, while its empirical ratio ensures correct stoichiometric dosing in formulations. The shift from empirical to molecular is, therefore, not merely a notational upgrade, but a leap in mechanistic understanding—vital for innovation in synthetic chemistry and materials design.
The distinction gains practical significance in analytical rigor. Empirical formulas serve as frontline tools where precision demands minimal structural assumption—ideal for treating unknowns. Molecular formulas, in contrast, are the compass for targeted applications, guiding synthesis, characterization, and performance prediction.
Whether quantifying pollutant degradation or designing a polymer with specific properties, choosing the right formula depends on the question at hand.
Empirical vs. molecular formulas reflect two complementary facets of chemical language—one rooted in stoichiometric necessity, the other in structural revelation.
Together, they form an unassailable framework for modern chemistry, ensuring accuracy from mass balance to molecular blueprint. As analytical tools advance, this duality remains central: empirical for clarity, molecular for complexity.
In the end, neither formulation dominates outright; instead, their synergy empowers chemists to ask deeper questions, verify results rigorously, and unlock new frontiers—proving that in chemistry, form is as vital as function.


Related Post

Netflix’s Sugarcane: A Haunting Chronicle of Trauma, Silence, and Silenced Histories

Bus Simulator Indonesia: Unlock Easy Money Hacks That Turn Commute Time Into Cash

Alamat Telinga Berdenging Terus: Tinitus Signs and the Silent Struggle to Break Tinnitus Chains
/IUSClockTower-56a5bd745f9b58b7d0de45aa.jpg)
What Time Zone Is Louisville, KY In? The Precise Time Zone Shifting Louisville Occupies

