Erythroblasts with Pappenheimer Bodies: Hidden Clues in Red Blood Cell Development
Erythroblasts with Pappenheimer Bodies: Hidden Clues in Red Blood Cell Development
In the intricate landscape of hematopoiesis, erythroblasts represent a pivotal stage in red blood cell formation, where protoerythrocytes mature through a series of morphological and biochemical transformations. Among the most revealing features observed in these developing cells are Pappenheimer bodies—distinctive cytoplasmic inclusions uniquely tied to iron metabolism and erythroid differentiation. Their presence, visible under light microscopy, serves as a diagnostic hallmark in specific blood disorders and offers critical insights into the molecular machinery governing erythropoiesis.
Understanding erythroblasts with Pappenheimer bodies demands a deep dive into their structure, formation, and clinical significance.
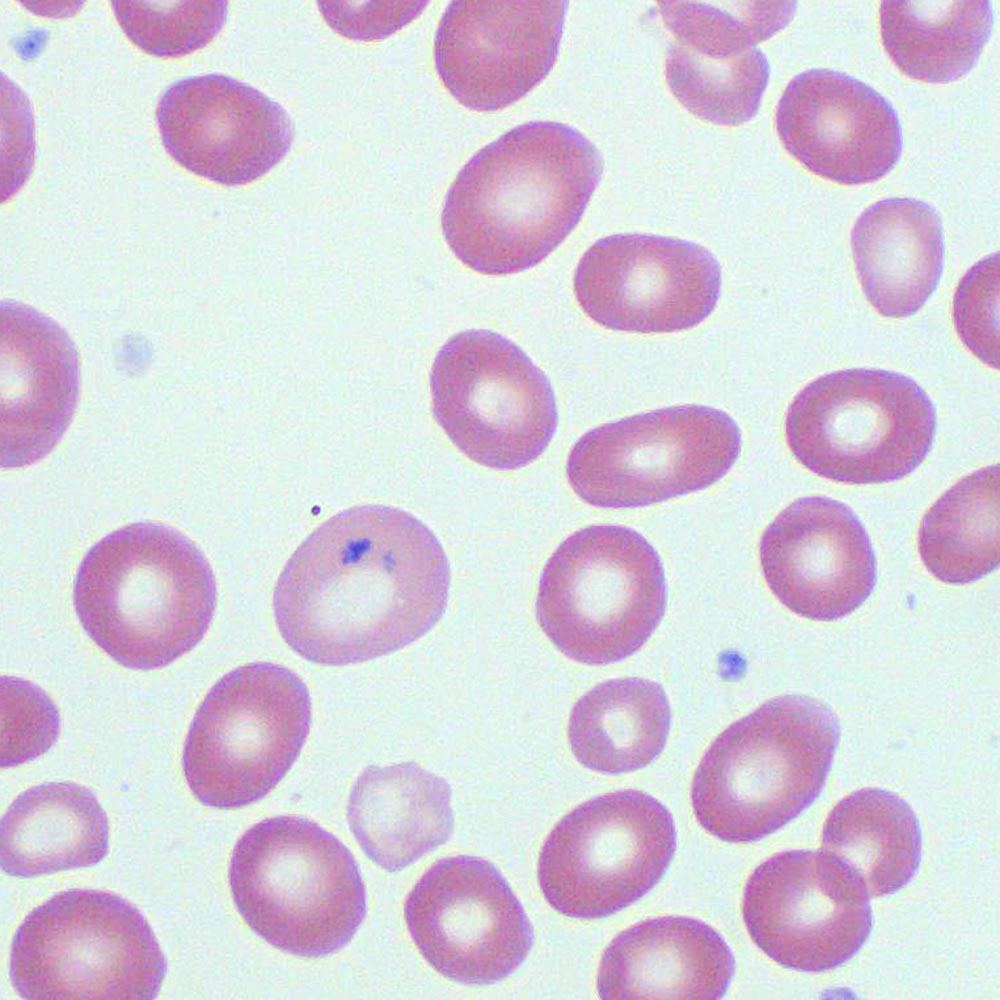
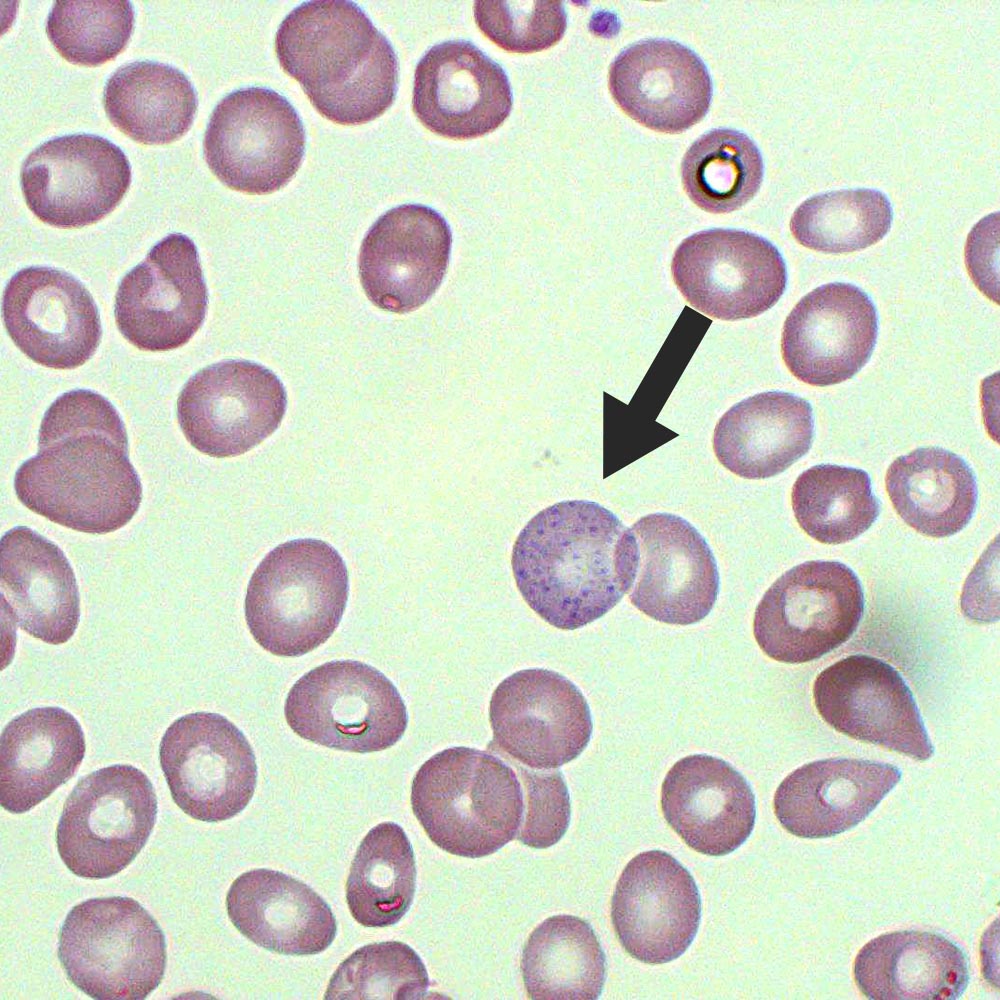
Unlike other blood cell precursors, these immature erythroblasts exhibit accumulations of ribosomal RNA-bound iron granules—Pappenheimer bodies—that appear as coarse, dark reddish-brown inclusions clustered within the cytoplasm. These sãoocytes, primarily found during the synchronized erythroblast maturation phase, reflect the cell’s readiness to synthesize hemoglobin and carry out terminal differentiation.
The Morphology and Biochemistry of Pappenheimer Bodies
Pappenheimer bodies are not mere artifacts but biologically meaningful structures composed of crude RNA, ribosomes, and iron-loaded transferrin complexes. Their formation is orchestrated by the depolarization of iron handling pathways in erythroblasts, where excess or improperly regulated iron accumulates within cytoplasmic vesicles.
Originally described in the 1920s by German pathologist Ludwig Pappenheimer, these inclusions were initially linked to inherited disorders affecting iron homeostasis.
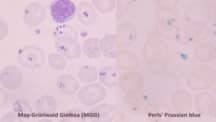
- Composition: The core consists of ribosomal RNA bound to iron-binding proteins, forming semi-lipid granules that stain intensely with Prussian blue, confirming iron presence.
- Localization: Typically found in the terminal erythroblasts (normoblasts), particularly during the polychromatophilic and reticulocytes stages, indicating active protein and lipid synthesis.
- Functional Role: Though once considered passive storage sites, current research suggests Pappenheimer bodies may regulate localized iron delivery to emerging hemoglobin molecules, balancing iron availability with oxygen-dependent biosynthesis.
Erythroblast Development and the Appearance of Iron Inclusions
Erythroblasts progress through a tightly regulated sequence from proerythroblast to normoblast, culminating in the release of reticulocytes. During terminal maturation, erythroblasts undergo nuclear extrusion, extensive cytoplasmic condensation, and organelle reduction—processes that coincide with the emergence of Pappenheimer bodies.
This timing is crucial: their appearance marks a metabolic shift toward heme synthesis and red pigment deposition.
At the heart of this transformation lies the erythroid lineage’s demand for iron. Hemoglobin biosynthesis requires iron incorporation into protoporphyrin IX, a process modulated by iron-sulfur cluster assembly and mitochondrial iron export. When these systems falter—due to genetic defects, nutritional deficiency, or inflammatory conditions—excess unbound iron accumulates, forming Pappenheimer bodies.
Their visibility under routine Wright-Giemsa-stained smears makes them objective markers for diagnosing iron dysregulation within the bone marrow.
Clinical Significance and Diagnostic Insights
Among hematopathology’s toolkit, Pappenheimer bodies serve as both biomarker and window into erythroid dysfunction. Their presence is most prominent in:
- Iron-refractory iron deficiency anemia (IRIDA), where genetic mutations impair iron sensing and utilization.
- Sideroblastic anemias, characterized by defective heme synthesis and compensatory iron trapping.
- Aplastic anemia, where bone marrow failure exposes immature erythroblasts to disrupted iron metabolism.
- Certain myelodysplastic syndromes, reflecting dysplastic maturation and cytoskeletal iron mislocalization.
While not diagnostically definitive on their own, their correlation with elevated mean corpuscular hemoglobin index (MCHI), ringed sideroblasts, or elevated soluble transferrin receptor levels strengthens clinical correlation. Malunky morphological features such as coarse, granular-iron staining cytoplasm prompt further investigation into inherited or acquired iron-related disorders, guiding precise therapeutic interventions.
From Laboratory Findings to Patient Care
In practice, detecting Pappenheimer bodies transforms abstract cytology into actionable clinical intelligence.
Automated hematology analyzers may overlook subtle inclusions, but skilled peripheral blood smear reviewers recognize them as telltale signposts of underlying metabolic strain. When combined with iron studies—serum ferritin, TIBC, ferritin ratios—they illuminate a patient’s erythropoietic capacity and iron homeostasis state. For clinicians managing anemias or bone marrow pathologies, identifying these granules elevates diagnostic precision, enabling early intervention and tailored management strategies.
Advancing beyond mere observation, ongoing research probes whether manipulating Pappenheimer body formation—via modulating iron trafficking proteins like DMT1 or ferritin—could mitigate erythroid stress in chronic disease.
While such therapies remain experimental, the inclusive nature of these granules continues to bridge basic science and clinical hematology, revealing how nanoscale inclusions encode vital truths about blood cell health.
In the ceaseless quest to decode blood disorders, erythroblasts bearing Pappenheimer bodies stand as microscopic sentinels—silent but significant, invisible to the untrained eye yet indispensable in the meticulous science of hematopoiesis. Their iron-laden presence tells a story of development, trouble, and resilience, underscoring the profound complexity embedded within a single blood smear.
Related Post

Emily Ratajkowski’s Power and the “Blurred Lines”: When Art, Controversy, and Cultural Shock Collide

Parfum Sense Laverne in Tunisia: Affordable Elegance at a Price That Delights

Dr. Does Chemistry Exam: The Scientific Backbone Behind Answering Chemistry Questions with Precision

Can I Apply for Nin Online? Everything You Need to Know Before Submitting Your Digital Footprint

