How Many Protons Are in Oxygen? The Atomic Secret Behind a Life-Sustaining Element
How Many Protons Are in Oxygen? The Atomic Secret Behind a Life-Sustaining Element
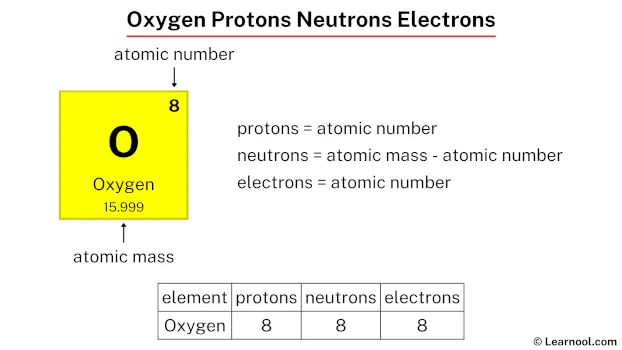
Oxygen, the second most abundant element in Earth’s crust and a vital component of life, holds a number of protons that defines its very identity: 8. This precise atomic number—8 protons—places oxygen in group 16 of the periodic table, among chalcogens like sulfur, selenium, and tellurium. Understanding the proton count is not merely an academic detail; it underpins how oxygen reacts, bonds, and sustains biological and industrial processes.
Every atom’s proton number is a fingerprint of its chemistry, shaping everything from breathable air to the water molecules that define our planet.
At the core of every oxygen atom are exactly 8 protons, a figure that determines its position and behavior in the periodic system. This number arises from the fundamental structure of the atom: protons are positively charged particles in the nucleus, balancing the negative charge of electrons to maintain atomic stability.
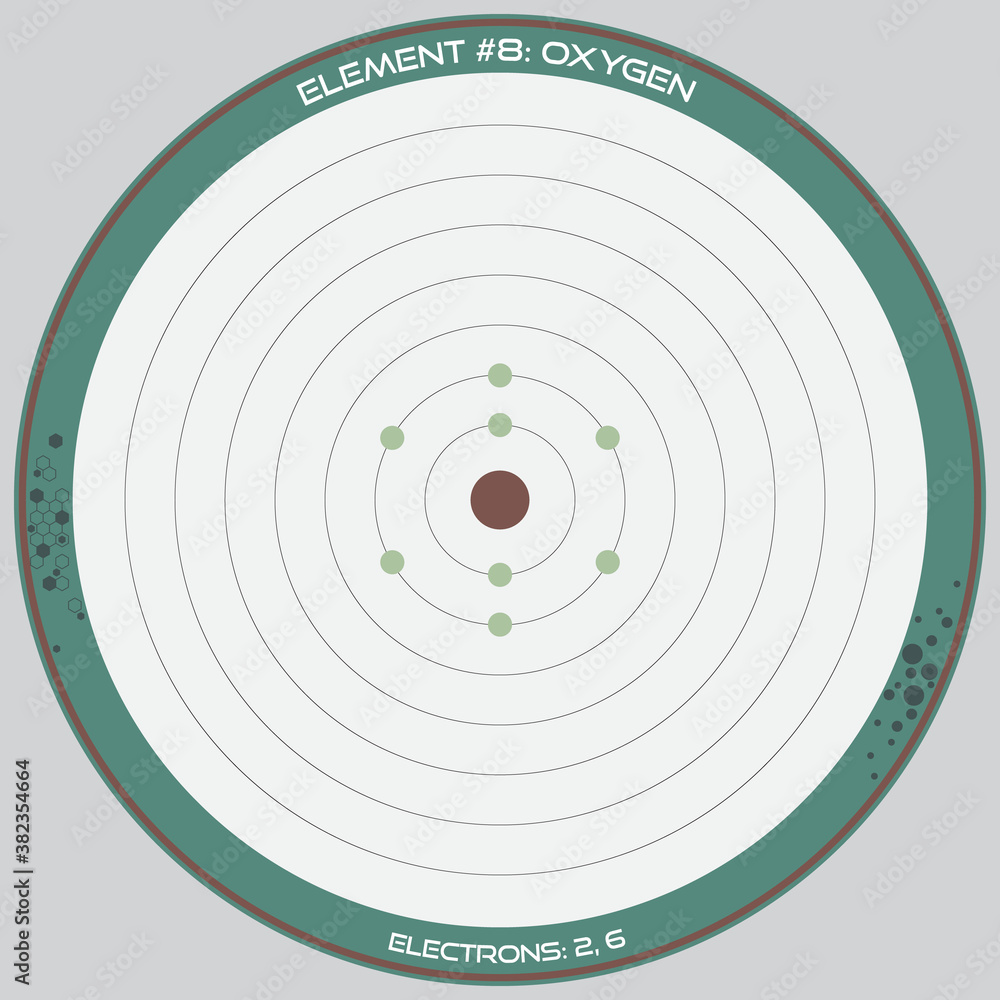
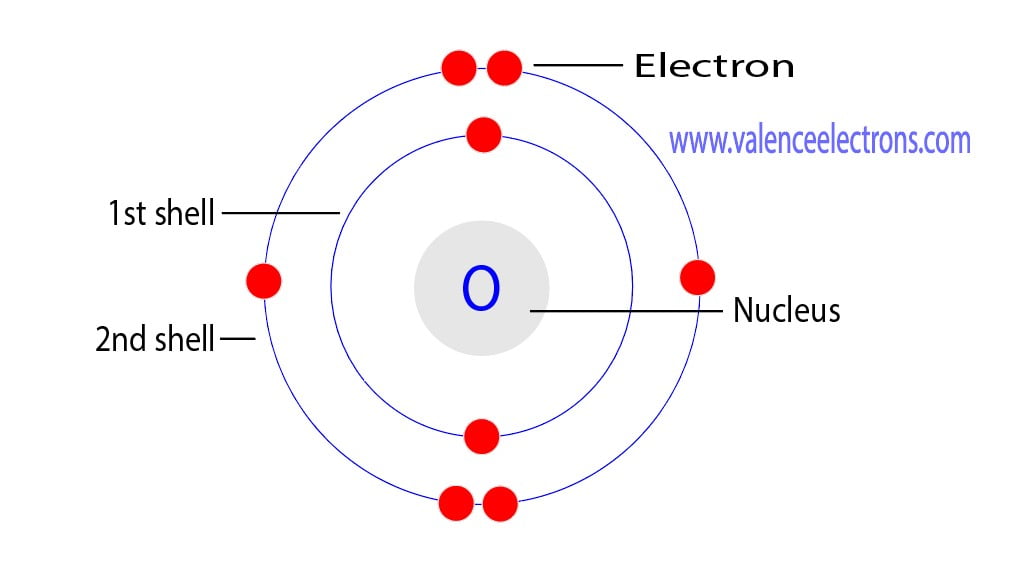
In oxygen’s case, 8 protons combine with 8 electrons (in a neutral atom) and a multitude of neutrons—typically around 8 or 10 depending on the isotope—to form the element’s total atomic weight of approximately 16.
Oxygen’s proton count directly influences its chemical reactivity. With six electrons filling the nearest energy shells, oxygen seeks to gain two more electrons to achieve a stable electron configuration, forming diverse compounds like water (H₂O), carbon dioxide (CO₂), and ozone (O₃).
This electron configuration, enabled by 8 protons, allows oxygen to act as a powerful oxidizing agent—capturing electrons in redox reactions essential to combustion, respiration, and industrial synthesis.
Among the 18 elements in group 16, oxygen stands out for its high electronegativity—a direct consequence of its 8-proton core. Electronegativity, a measure of atomic pull on shared electrons, explains why oxygen bonds strongly with hydrogen, carbon, and metals.
In H₂O, for instance, oxygen’s proton number enables polar covalent bonds that give water its unique solvent properties and ability to support life.
Isotopic variation does not alter the number of protons; oxygen naturally occurs in several isotopes—most commonly ¹⁶O, ¹⁷O, and ¹⁸O—without changing its atomic number. While isotopes differ in neutron count and mass, they retain identical proton cores, meaning oxygen’s chemical behavior remains consistent across varieties.
This stability underscores that proton count, not neutrons, governs the element’s identity.
Beyond biology, oxygen’s proton architecture fuels critical industrial and environmental systems. In metallurgy, oxygen is used to refine metals by removing impurities through oxidation.
In environmental science, atmospheric oxygen (O₂), defined by eight protons per atom, enables aerobic respiration in organisms and drives atmospheric chemistry, including the formation and breakdown of the ozone layer.
From ancient alchemists to modern quantum chemists, the number of protons in oxygen has guided discoveries for centuries. This simple atomic figure is far more than a statistic—it is the root of oxygen’s dual role as both a breath of life and a silent architect of change across Earth’s systems.
Understanding how many protons define oxygen enriches not only scientific knowledge but deepens our appreciation for the element’s invisible yet indispensable influence on every living process.

Related Post

From Rio to Global Domination: Decoding the Cultural Power Behind “Despacito” Lyrics

Discover Jackson Hole Through Its Heart: A Deep Dive Using the Interactive Location Map

Stop Running Woo Lotti Vid: Unveiling the Viral Story Behind the Intriguing “Eo”

Ethel Kennedy Net Worth: A Deep Dive Into Her Life and Legacy

