How to Precisely Determine the Empirical Formula: A Step-by-Step Scientific Guide
How to Precisely Determine the Empirical Formula: A Step-by-Step Scientific Guide
To unlock the invisible identity of a chemical compound, scientists rely on the empirical formula—a concise yet powerful descriptor of the simplest whole-number ratio of atoms within a molecule. This formula reveals not only elemental composition but also the stoichiometric relationships that govern chemical behavior, serving as a foundational tool in laboratories worldwide. Learning how to determine an empirical formula combines analytical precision with methodical chemistry, bridging theory and real-world application in ways that unlock deeper insight into material science, pharmaceuticals, and environmental testing.
The Empirical Formula: Definition and Significance
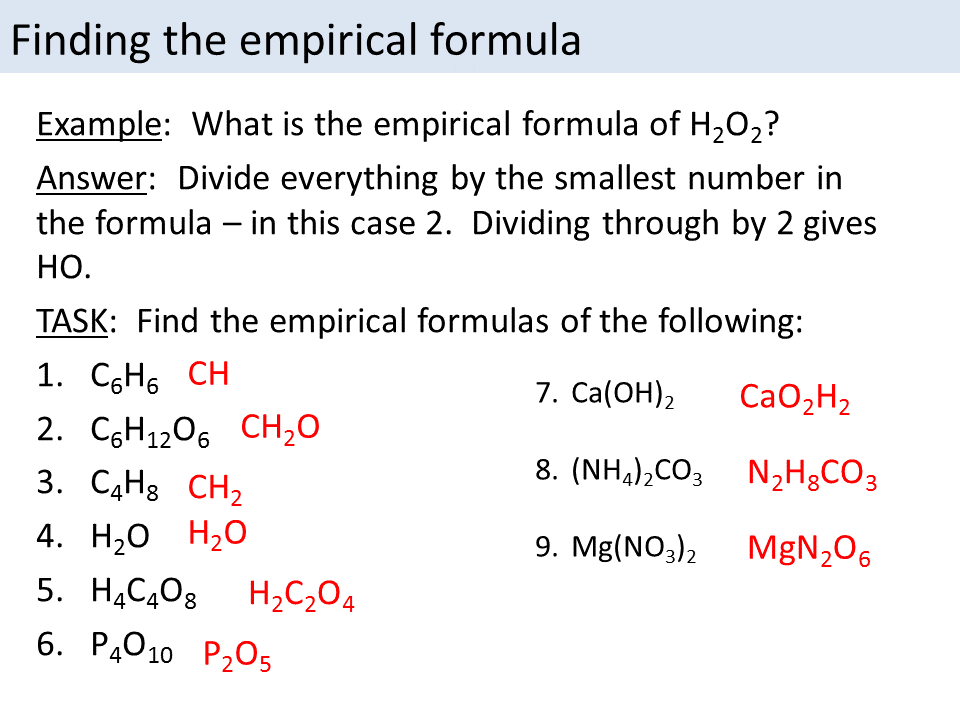
An empirical formula represents the relative number of each type of atom in a compound using the fewest whole-number ratios, stripped of molecular complexity. Unlike molecular formulas that disclose all atoms—including those in charge carriers like oxygen in metal oxides—empirical formulas reflect the core atomic skeleton. This distinction is vital: “Consider sodium chloride,” explains chemist Dr.Elena Marquez, “it’s NaCl in molecular form, but its empirical formula simplifies to Na:Cl in ratios reflecting actual bonding proportions.” This stripped-down representation enables accurate quantification in stoichiometry, enabling chemists to calculate reactant quantities, predict product yields, and validate synthetic pathways. Understanding empirical formulas underpins key scientific processes, from formulating pharmaceuticals in precise dosages to analyzing pollutants in water systems. Their utility extends to academic research, industrial chemistry, and quality control, where reliability and accuracy are non-negotiable.
Step-by-Step Process: Measuring Atoms and Calculating Ratios
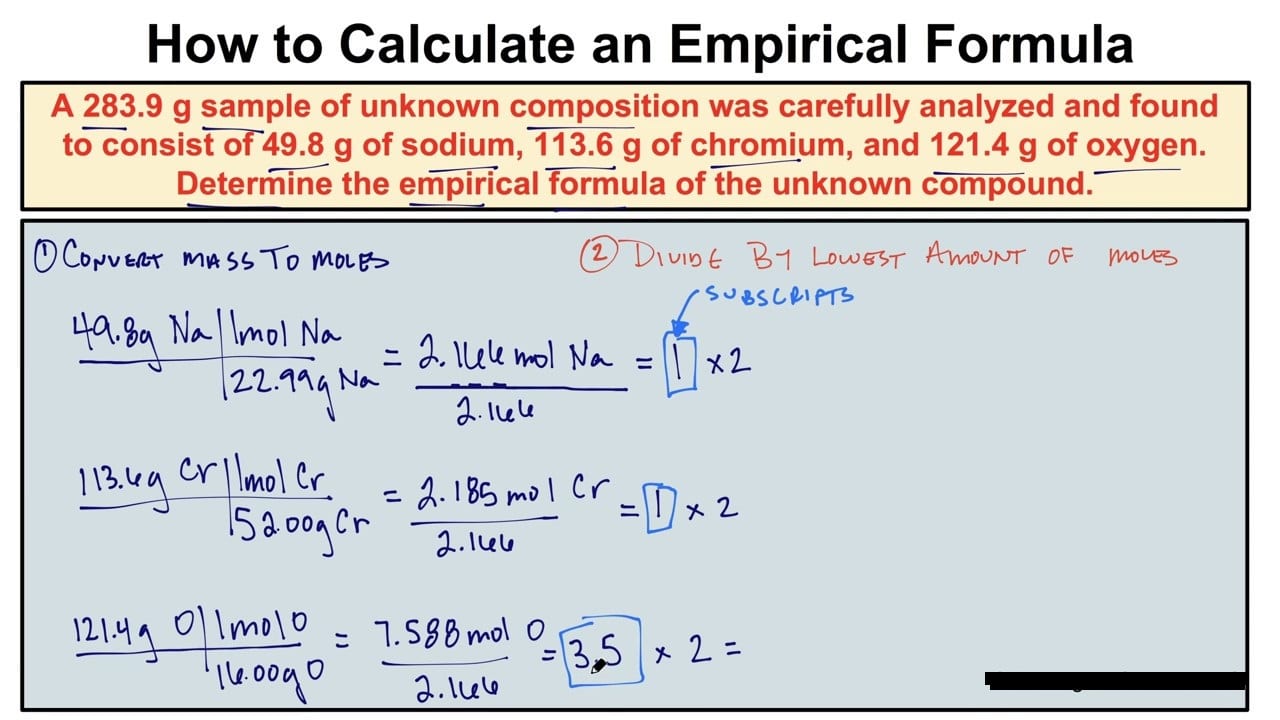
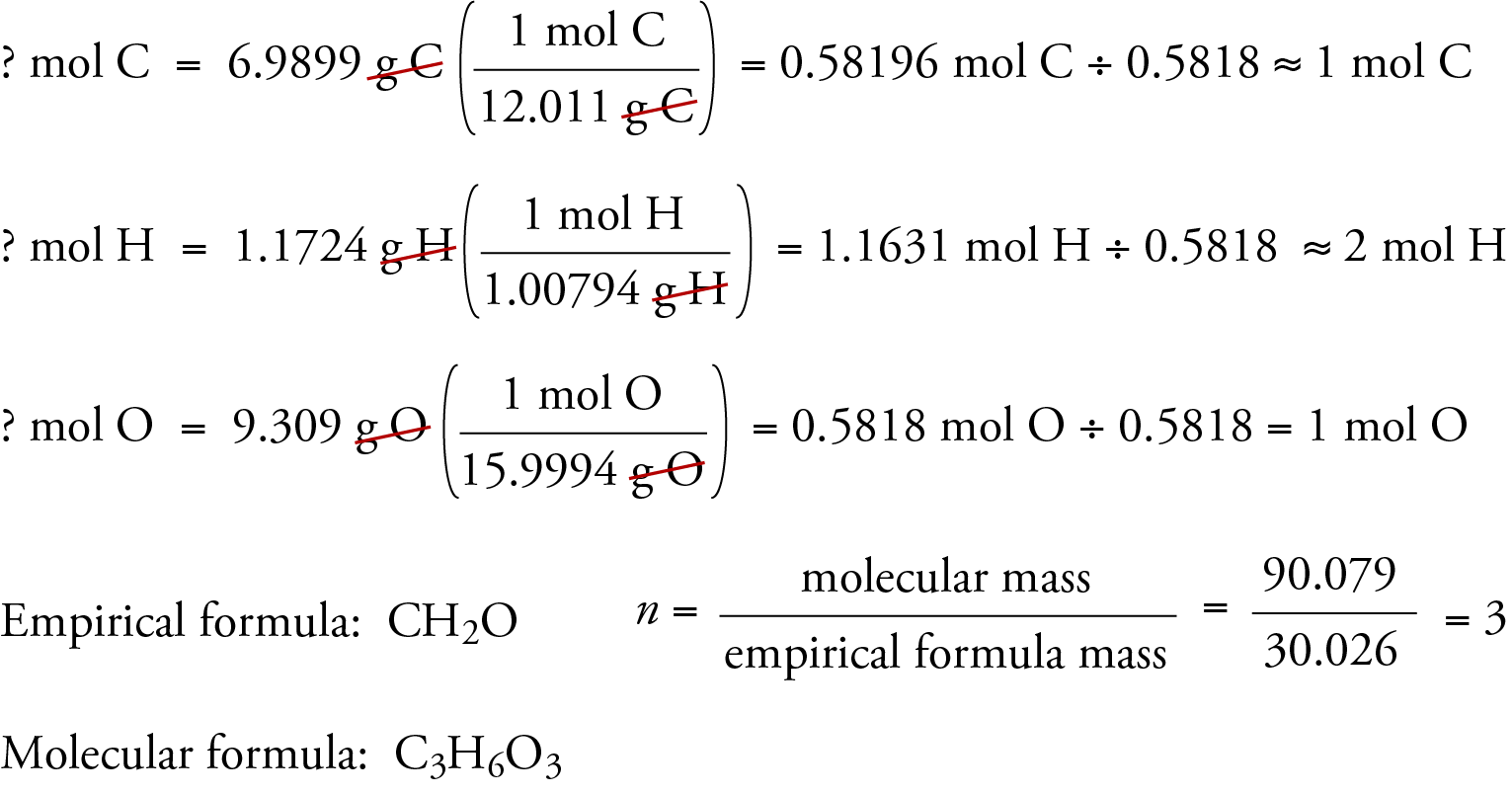
Determining an empirical formula begins with definitive atomic quantity measurements. These quantities are collected through precise laboratory techniques such as gravimetric analysis, spectrometry, or titration—each chosen based on the sample type and available instrumentation. The primary data sources include: - Mass harvested after complete reaction (e.g., residue after combustion or precipitation) - Elemental percentages derived via analytical methods like CHN analyzers or X-ray fluorescence - Molar proportions when pure samples are analyzed using modern instrumental tools such as mass spectrometers Once accurate mass or percentage data are secured, the next step is converting percentages into moles.“This conversion is non-negotiable—empirical formulas depend on molar relationships,” emphasizes analytical chemist James Reed. “Percent composition alone isn’t enough; dividing by atomic weights yields the mole ratios essential for finding the simplest integer ratio.” For example, if a sample contains 40% carbon, 6.7% hydrogen, and 53.3% oxygen by mass, the first step is converting these percentages into moles: - Carbon: 40 g ÷ 12.01 g/mol ≈ 3.33 mol - Hydrogen: 6.7 g ÷ 1.008 g/mol ≈ 6.65 mol - Oxygen: 53.3 g ÷ 16.00 g/mol ≈ 3.33 mol Dividing each by the smallest mole value (3.33 in this case) gives the mole ratio: - C: 3.33 ÷ 3.33 = 1 - H: 6.65 ÷ 3.33 ≈ 2 - O: 3.33

Related Post

Where Is Omaha? Unlocking the Strategic Heart of the American Midwest

WhatIs1500InMilitaryTime? Decoding the 1500-Based Code Used in Modern Military Systems

Unveiling The Legacy And Impact Of Ottavia Busia, Ariane Bourdain, And Anthony Bourdain

Download Logo Kemenag Terbaru 2022 PNG Instantly: Free High-Quality Design at Your Fingertips

