LyticCycleVsLysogenic
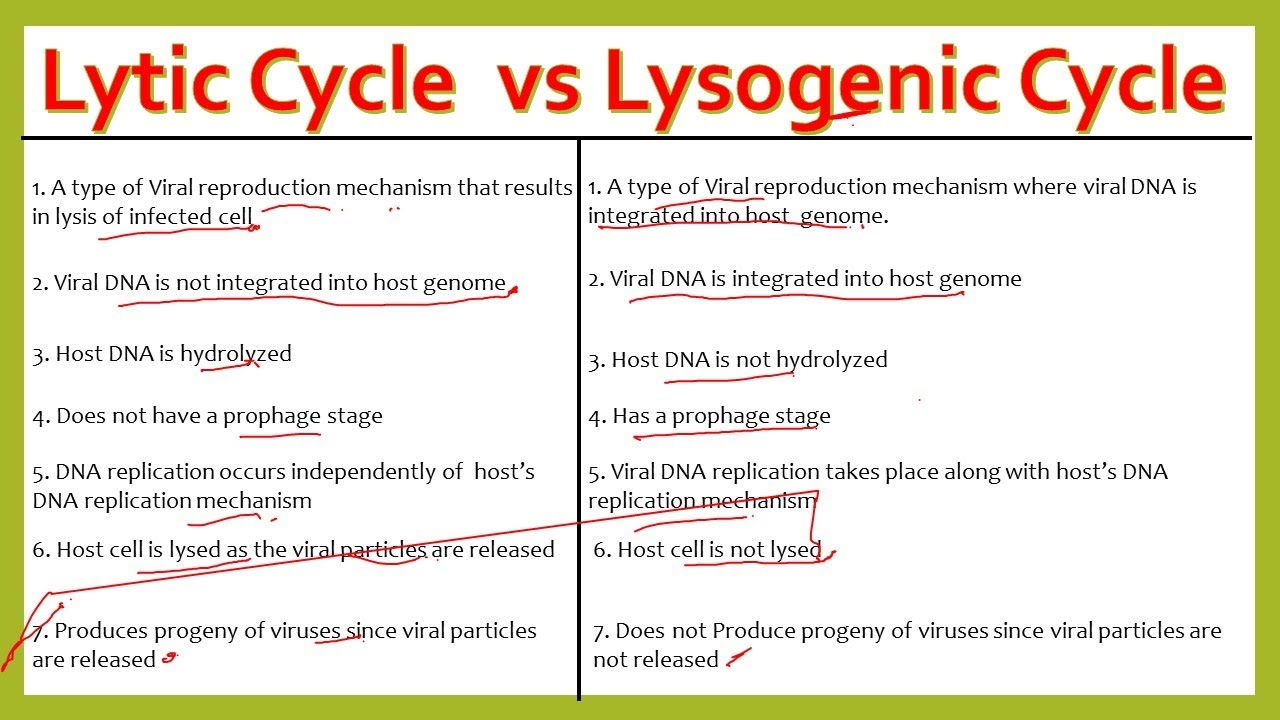
When viruses wrestle with bacterial life, two distinct survival strategies emerge: the lytic cycle and the lysogenic cycle—opposing pathways that determine whether a viral encounter ends in cellular destruction or stealthy integration. Understanding the lytic cycle versus the lysogenic cycle reveals the fundamental choices viruses make in shaping microbial ecosystems, driving evolution, and influencing human health. While the lytic cycle unleashes explosive viral replication and immediate host lysis, the lysogenic cycle offers a latent, often hidden existence, freezing viral DNA within bacterial genomes for generations.
This contrast illuminates not just virology, but the delicate balance of destruction and coexistence in nature.
The Lytic Cycle: A Violent Microbial Takeover
The lytic cycle represents a swift, aggressive mode of viral propagation engineered for rapid replication. In this aggressive pathway, bacteriophages inject their genetic material into host bacteria, commandeering cellular machinery to produce new virus particles. Once adequately assembled, the host cell ruptures—lyses—releasing hundreds of progeny viruses to infect neighboring cells.This cycle is both efficient and devastating, often causing death within hours or days. Key stages of the lytic cycle include: - Attachment: The phage binds precisely to specific receptors on the bacterial surface. - Injection: Viral nucleic acid enters the host cell while the protein coat remains outside.
- Biosynthesis: The host’s ribosomes and enzymes churn out viral DNA, proteins, and capsids. - Maturation: New viral components assemble into complete genome-containing virions. - Release: Host cell lysis occurs, typically via enzymes like holins and endolysins, broadcasting virions into the environment.
Lytic phages, such as T4 bacteriophage, exemplify this assault strategy. Their rapid replication rate makes them powerful agents of bacterial mortality, critical in regulating bacterial populations in oceans, soil, and the human microbiome. Environmental stresses like nutrient depletion or UV exposure often trigger the switch from dormant to lytic states, turning phages into ecological forces of destruction.
Real-World Impacts of the Lytic Cycle
In natural ecosystems, lytic phages act as nature’s switches—limiting bacterial blooms and maintaining microbial diversity.Their role is vital in marine environments, where phage-driven lysis recycles carbon and nutrients, fueling the base of oceanic food webs. In agriculture, lytic phages are explored as alternatives to antibiotics for controlling bacterial pathogens in crops. Additionally, in medicine, engineered lytic phages hold promise in phage therapy—targeted destruction of antibiotic-resistant bacteria without harming human cells.
The Lysogenic Cycle: Silent Viral Integration
In contrast, the lysogenic cycle embodies patience and strategic integration.Instead of immediate destruction, the phage genome—now termed a prophage—falters into the host’s chromosome, replicating silently alongside bacterial division. This latent phase can persist for countless generations, lethargic but stable. Unlike the lytic route, the lysogenic cycle does not cause host death initially; the viral DNA remains dormant, often conferring new traits—such as toxin production or antibiotic resistance—enhancing bacterial adaptability.
The switch between lysogeny and lysis is regulated by complex genetic circuits, most famously exemplified by the lambda phage. Environmental cues—stress, DNA damage, or nutrient scarcity—can induce the prophage to exit dormancy, initiating the lytic program. This flexibility offers a survival advantage: in stable, nutrient-rich environments, lysogeny allows viruses to passively propagate without exports.
Examples of lysogenic integration include Staphylococcus aureus harboring prophages encoding toxic mini-cells, significantly increasing virulence. Similarly, many pathogenic bacteria encode virulence factors acquired through lysogenic conversion, transforming harmless strains into medical threats. This ability to carry and transfer genetic payloads makes lysogeny a cornerstone of horizontal gene transfer and microbial evolution.
Strategic Advantages and Evolutionary Significance
The lysogenic cycle’s silence is its strength. By avoiding detection and destruction, latent phages persist unseen—immune to immune attacks and antibiotics designed for active replication. Deciphering this stealth mode offers insight into viral persistence in chronic infections and bacterial gene network rewiring.From an evolutionary perspective, lysogeny enables long-term cooperation between phages and hosts. Horizontal gene transfer via prophages accelerates bacterial adaptation, allowing rapid spread of advantageous genes across populations. Moreover, lysogeny fuels genetic diversity, driving innovation in microbial genomes that shapes ecosystems and human health alike.
Yet, this subtlety masks risk. Inducible lysogenic prophages can trigger sudden outbreaks—when stress disrupts dormancy, causing mass lysis and disease flares. In antibiotics, stressed bacteria may awaken dormant phages, releasing virulent progeny and complicating treatment.
Divergent Outcomes, Shared Importance
The lytic and lysogenic cycles represent two ends of a viral survival spectrum—destruction versus concealment—each vital to microbial ecosystems. While lytic cycles drive swift turnover and population control, lysogeny fosters stealth, adaptation, and innovation. Neither strategy dominates universally; their prevalence depends on environmental conditions and host biology.This balance underscores a deeper truth in virology: nature favors versatility. Phages switch between modes dynamically, responding to invisible environmental cues. This adaptability shapes microbial communities, influences antibiotic resistance spread, and fuels evolutionary leaps across domains of life.
Understanding lytic and lysogenic pathways is not just about viral mechanisms—it’s about unraveling the invisible forces that govern bacterial dominance, ecosystem health, and medical progress. As science advances, harnessing the precision of phage behavior in both cycles promises revolutionary tools in medicine, agriculture, and bioremediation, proving that even the smallest viral battles carry outsized influence.



Related Post

First Invention Of Tv

The Rise of FootballBros: Where Passion Meets Community in the Modern Football Landscape

Master Snapchat Account Management: How to Take Full Control of Your Login Experience

Is Mist Pot Cit Safe During Pregnancy? What You Need to Know Before Using This Common Remedy

