Mass Definition Chemistry: The Atomic Blueprint That Shapes Every Reaction
Mass Definition Chemistry: The Atomic Blueprint That Shapes Every Reaction
At the core of every chemical transformation lies a definition so foundational yet often misunderstood: mass in chemistry is not merely a measure of weight—it is the molecular mass that governs stoichiometry, reaction kinetics, and the very identity of substances. Mass Definition Chemistry bridges theory and practice, explaining how precise atomic masses determine how elements combine, transform, and interact. This concept is the cornerstone of quantitative analysis, underpinning everything from pharmaceutical development to industrial manufacturing.
Understanding mass at the atomic level clarifies how chemists predict reaction outcomes, calculate yields, and define molecular structures with precision.
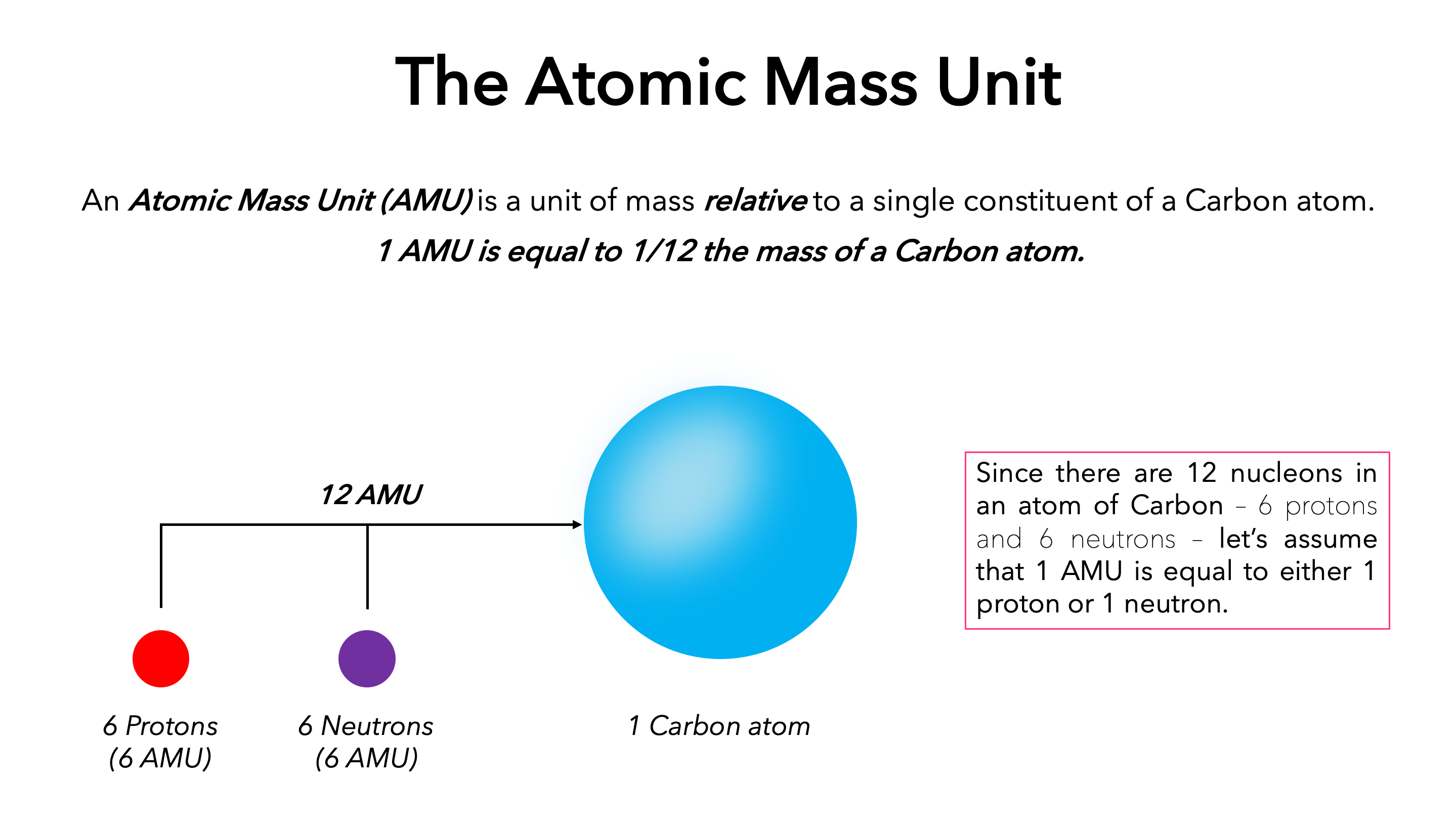
Mass, defined as the amount of matter an object possesses—commonly expressed in grams or atomic mass units (amu)—is central to Mass Definition Chemistry. The atomic mass unit (amu), introduced by Harold Urey in 1931, standardizes comparison by relating mass to carbon-12, where 1 amu equals precisely one-twelfth the mass of a single carbon-12 atom.
This system allows scientists to express the mass of atoms, molecules, and compounds with extreme accuracy, forming a universal language for chemical quantification. “Mass is the bridge between the macroscopic world we observe and the microscopic domain of atoms,” explains Dr. Elena Martinez, a senior chemist at the Max Planck Institute.
“Without precise mass values, the predictive power of chemistry collapses.”
At the heart of Mass Definition Chemistry is the molecular mass, derived from summing the atomic masses of all atoms in a molecule. For example, water (H₂O) has a molecular mass of ~18.015 amu—calculated as (2 × 1.008 amu for hydrogen) + (16.000 amu for oxygen). Organic compounds, with carbon, hydrogen, oxygen, nitrogen, and sulfur components, require careful summation.
Ethanol (C₂H₅OH) has a molecular mass of approximately 46.07 amu: (2 × 12.011) + (6 × 1.008) + (1 × 16.00) + 16.00. This mass is indispensable for determining stoichiometric ratios in synthesis. “Every molecular formula translates directly into measurable mass, enabling chemists to scale reactions with confidence,” notes Dr.
James Lin, a quantum chemist at MIT.
Mass Definition Chemistry also governs reaction kinetics, where precise molecular mass influences collision frequency and activation energy. Lighter molecules travel faster at a given temperature (per Graham’s law), accelerating reactions in processes like catalytic conversion in automotive exhaust systems. Moreover, in mass spectrometry—an analytical technique central to modern chemistry—mass-to-charge ratio (m/z) measurements identify molecules by their exact mass.
A carbon-based impurity with molecular mass 44.01 versus 43.01, for instance, yields distinguishable peaks, enabling detection at trace levels. “Mass spectrometry transforms mass into a narrative of molecular identity,” says Dr. Priya Kapoor, a forensic chemist.
“One extra proton, or a missing hydrogen, tells a story of structural variation.”
Beyond analysis, mass coordinates synthetic design. When designing a reactor for ammonia production via the Haber process, engineers rely on precise mass balance to optimize input gases—nitrogen and hydrogen—ensuring maximum yield with minimal waste. Similarly, pharmaceutical developers use Mass Definition Chemistry to calculate exact dosages based on molecular weight, where even a single gram differential can affect efficacy or toxicity.
Vitamin C (C₆H₈O₆), with a molecular mass of 176.12 g/mol, must be accurately dosed to meet biological requirements without adverse effects.
Mass Definition Chemistry also enables environmental monitoring. By tracking isotopic abundances—variations in atomic mass due to neutron count—scientists trace pollution sources. Carbon-13 vs.
carbon-12 ratios reveal fossil fuel contributions to atmospheric CO₂, informing climate policy. In geochemistry, oxygen isotope mass differences detect ancient water sources, shedding light on planetary history. “Every atom carries a story written in its mass,” remarks Dr.
Luca Rossi, isotope geochemist at the University of Rome. “Mass is not static—it’s a dynamic indicator of process and origin.”
For students and professionals alike, mastering Mass Definition Chemistry is essential. It transforms abstract formulas into tangible quantities, turning theoretical equations into practical solutions.
From predicting reaction yields to ensuring safe medication dosing, precise mass measurement lies at the intersection of science and application. In every laboratory, factory, and analysis, mass remains the silent architect—defining what happens, when it happens, and why it matters. As analytical techniques advance and global challenges demand ever-greater accuracy, Mass Definition Chemistry remains not just a foundational concept, but a living, evolving science that shapes the future of chemistry across industries and disciplines.



Related Post

Elevate Growth: Aceleration City Is Redefining Urban Innovation
Unlock Seamless Efficiency: The Ultimate Guide to PES 6 Configuration

Unveiling the Life of Dani Daniel: A Living Ministry That Transcends Boundaries

Wordle.Clue: Decoding the Words Behind Today’s Most Challenging Clues

