Molar Mass Copper: The Atomic Precision Behind a Global Industrial Cornerstone
Molar Mass Copper: The Atomic Precision Behind a Global Industrial Cornerstone
Copper, a metal etched in human history from ancient civilizations to modern high-tech applications, remains a linchpin in global industry—its utility deeply rooted not just in conductivity, durability, and malleability, but in the precise atomic foundation defined by molar mass. With a molar mass of 63.546 g/mol, copper’s atomic identity governs everything from alloy formulation to precision manufacturing. Understanding this fundamental metric unlocks insight into why copper persists as indispensable material across sectors including electronics, architecture, renewable energy, and medical devices.
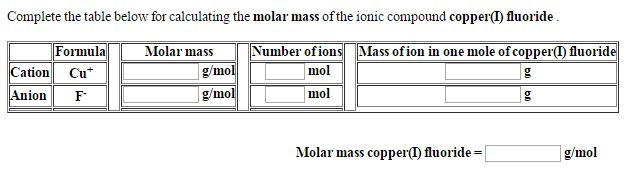
The molar mass of copper—63.546 grams per mole—reflects the precise sum of its atomic weight (linked to its 29 protons, 34 neutrons, and 29 electrons). This value is not arbitrary; it underpins chemical behavior, stoichiometric calculations, and material performance. “The consistency of copper’s molar mass ensures predictable electrochemical and thermal properties,” notes Dr.
Elena Torres, a materials scientist at the Copper Development Association. “This predictability is critical for engineering applications where reliability is non-negotiable.” Copper’s atomic structure enables it to conduct electricity efficiently—among the highest of all metals—with electrons free to move within a crystalline lattice. This property is quantified in terms of resistivity, but its origin lies in the atomic mass and electron configuration.
At 63.546 g/mol, copper balances moderate atomic weight with a high density (8.96 g/cm³), contributing to its weight efficiency in load-bearing and conductive components. Alloys like brass (copper-zinc) and bronze (copper-tin) are designed precisely around copper’s molar mass to tune strength, corrosion resistance, and workability.
In industrial chemistry, molar mass governs reaction stoichiometry.
For example, in electroplating, the amount of copper deposited relies on moles of Cu²⁺ ions—calculated via mass and molar mass to ensure thin, uniform coatings on semiconductor wafers or electrical connectors. Similarly, copper oxide production and surface oxidation rates depend on atomic quantity and mass, influencing corrosion protection strategies in infrastructure.
Copper’s role extends beyond traditional uses into cutting-edge technology.
With the global push toward renewable energy and electric mobility, copper’s high conductivity—anchored in its atomic precision—makes it essential for power distribution systems, solar panel wiring, and electric vehicle motors. “As demand surges for clean energy infrastructure, copper’s molar stability ensures it remains the go-to material where performance meets scalability,” says industry analyst Raj Patel. The metal’s predictable behavior at scale supports reliable circuitry in everything from microchips to wind turbine generators.
Quality control in copper production hinges on exacting measurement of molar mass. Smelting, refining, and alloying processes are calibrated to maintain atomic consistency, ensuring uniform properties across batches. Deviations, however minor, can alter thermal expansion, electrical conductivity, or mechanical flexibility—critical in aerospace components or medical implants where precision cannot be compromised.
Mineral extraction and refining retain natural variations, but modern regions and smelters maintain tight tolerances around 63.546 g/mol. For instance, ergitive copper from Chilean deposits undergoes smelting processes calibrated to preserve atomic integrity, minimizing impurities that might disrupt the ideal atomic structure. This fidelity strengthens copper’s value in high-purity applications like semiconductors, where even trace impurities can degrade performance.
In summation, copper’s molar mass—63.546 g/mol—serves as more than a numerical constant; it is the factual backbone of the metal’s enduring utility across millennia. Through chemistry, engineering, and industrial innovation, this precise atomic value sustains copper’s reputation as a material engineered for reliability and future-ready performance. Whether in ancient coinage or advanced photovoltaic arrays, the atomic clarity of copper remains indispensable—a silent, steady force enabling progress across the modern world.


Related Post

Minji K of New Jeans: How a Centric Phrase is Propelling Her to Pop Stardom

Hollywood Movies Dubbed In Punjabi: Your Ultimate Guide to Global Stories in Your Mother Tongue
Top Spanish News Apps: Your Daily Fix for Accurate, Real-Time Spanish Language News

Watch Ovostreams Player: Revolutionize Live Sports Viewing with Seamless Online Events

