Osmosis Defined: The Life-Sustaining Process That Drives Cellular Function
Osmosis Defined: The Life-Sustaining Process That Drives Cellular Function
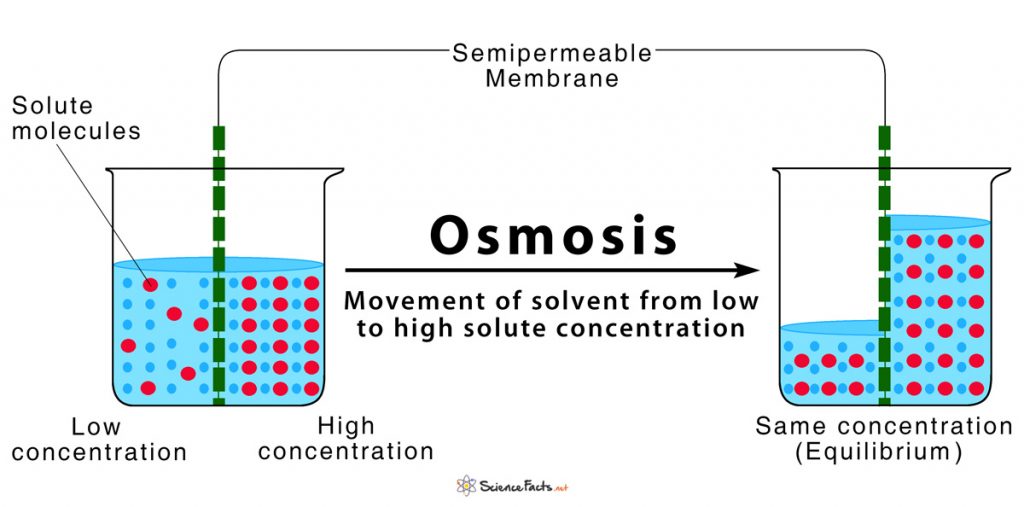
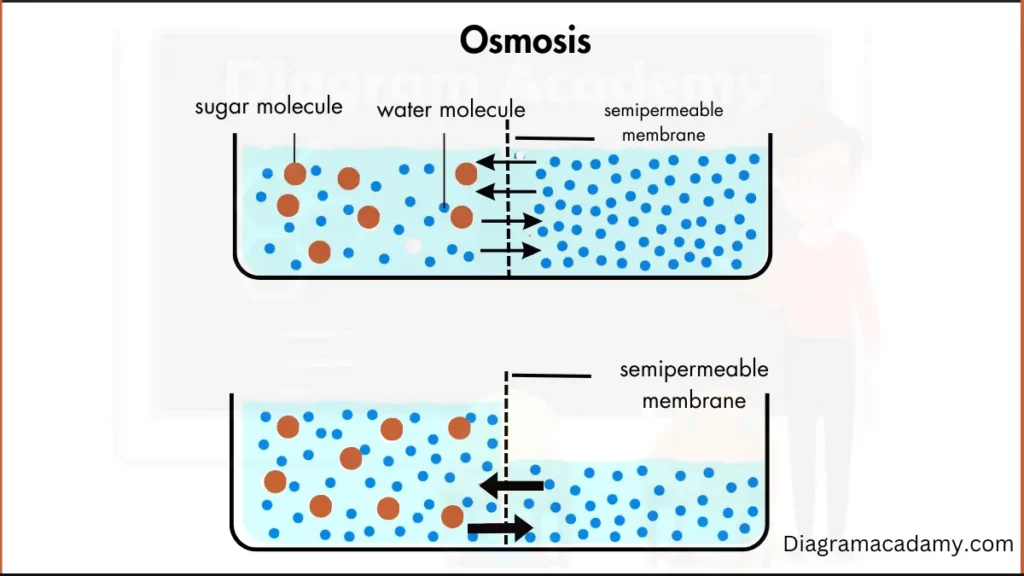
At its most fundamental level, osmosis is the passive movement of water across a selectively permeable membrane, driven by differences in solute concentration. This elegant yet powerful biological phenomenon governs how cells regulate volume, transport nutrients, and maintain homeostasis—making it indispensable to life at every scale, from microbial cells to human organs. “Osmosis, in essence, is water’s natural flow from dilute to concentrated environments,” explains Dr.
Elena Morales, a cellular biologist at the Institute for Biomedical Sciences. “It is not just a biochemical curiosity, but a foundational mechanism that sustains cellular health and function.”
Defined mathematically, osmosis describes the net movement of water molecules toward regions with higher solute concentration, counterbalanced by osmotic pressure—a force that opposes the natural diffusion gradient. This process occurs independently of active transport systems, relying solely on passive diffusion through lipid-bilayer membranes that permit water passage while excluding larger solutes.
The driving principle follows the movement of solvent—water—unlike general diffusion, where all particles move freely; osmosis specifically prioritizes water’s selective passage based on concentration gradients, a distinction critical for precision in biological systems. “Unlike simple diffusion, osmosis ensures that cells respond dynamically to their fluid environment without expending energy,” says Dr. Samuel Chen, a systems biologist at Stanford University.
“Cells don’t just absorb water—they control its rate, adapting instantly to changes in external pressure and solute levels.”
The Molecular Dance: Water Seeks Balance At the molecular level, osmosis operates through semi-permeable membranes—such as those found in cell walls and biological vesicles—that allow water molecules to cross while restricting ions, sugars, and proteins. In a hypotonic environment—where the outside fluid has lower solute concentration than inside a cell—water rushes inward to dilute the excess solutes, causing the cell to swell or even burst (lyse) if unchecked. Conversely, in a hypertonic setting, water exits the cell, leading to shrinkage (crenation).
Under isotonically balanced conditions, inflow and outflow stabilize, enabling optimal cellular function.
This equilibrium is vital in biological contexts. In plant cells, osmosis maintains turgor pressure—essential for rigid structure and efficient photosynthesis.
“Without osmotic balance, wilting would occur instantly,” notes Dr. Maria Lopez, a plant physiologist at the Agricultural Research Center. “Turgid cells support plant structure, enable nutrient transport, and sustain growth—all powered by osmotic regulation.” Animal cells, though lacking rigid walls, depend equally on osmotic control.
Kidney tubules, for example, fine-tune water reabsorption through osmotic gradients, ensuring proper blood volume and electrolyte balance.
Osmosis in Human Physiology: More Than Just Hydration
Beyond isolated cells, osmosis orchestrates large-scale physiological processes. The nephron, the functional unit of the kidney, relies on osmotic gradients to filter blood, reabsorb water, and concentrate urine.“In the loop of Henle, osmosis creates a gradient that enables water conservation,” explains Dr. Raj Patel, a nephrologist at Mayo Clinic. “This mechanism underpins our ability to survive in arid environments by minimizing water loss.”
Osmosis also governs cerebrospinal fluid (CSF) dynamics, maintaining intracranial pressure through precisely regulated water exchange between blood vessels and CSF-containing compartments.
Disruptions—such as in hydrocephalus, where fluid buildup results from impaired osmotic balance—highlight osmosis’s delicate role in neurological integrity. “Even minor shifts in solute concentration can trigger dangerous pressure changes,” warns Dr. Patel.
“Understanding osmosis here isn’t just academic—it’s clinical.”
Applications Beyond Biology: From Desalination to Smart Materials
The principles of osmosis extend far beyond cellular biology, influencing engineering, environmental science, and material innovation. Reverse osmosis, a technology mimicking the natural process, powers modern desalination plants by forcing seawater through semi-permeable membranes under high pressure, producing clean drinking water. “Nature’s osmosis pivot enabled this breakthrough,” asserts Dr.Chen. “We’ve harnessed it to solve global water scarcity, turning saltwater into life-saving hydration.”
In biological research and medicine, osmotic gradients guide drug delivery systems. Liposomes and nanoparticles exploit controlled water movement to release therapeutics precisely within targeted cells.
“We engineer osmotic triggers so drugs activate only where needed,” explains Dr. Elena Morales. “This specificity reduces side effects and increases efficacy—proof that osmosis, once debated in laboratories, now drives medical innovation.”
Balancing Act: Homeostasis at the Cellular Level
Maintaining osmotic homeostasis—or osmotic balance—is a critical function of maintaining internal stability, or homeostasis.Cells employ ion channels, transport proteins, and pump mechanisms to adjust solute concentrations dynamically. When external conditions shift, cells rapidly alter permeability or pump ions to restore equilibrium, preventing swelling or shrinkage. “Cells with intact osmotic regulation can buffer environmental stress,” Dr.
Morales states. “This adaptability is the cell’s first defense—about survival at the microscopic level, with macroscopic impacts on organismal health.”
Even microbial life depends on osmotic control. Bacteria adjusting to freshwater or saline environments rapidly modify membrane transporters, altering solute uptake and efflux to maintain cellular volume.
“Microbial osmosis is a straightforward but profound survival strategy,” says Dr. Chen. “It reveals osmosis as Nature’s default recipe for resilience—simple, precise, and universally vital.”
The Osmosis Legacy: A Fundamental Force Shaping Life
Osmosis is not merely a laboratory phenomenon or a textbook definition—it is a living, breathing force that orchestrates survival at every scale of existence.From single cells regulating internal pressure to entire ecosystems sustaining water balance, osmosis underpins the adaptive beauty of living systems. Its definition—simple in concept, infinitely complex in application—stands as a testament to biology’s elegance: nature’s passive mechanism, a silent river guiding life’s march forward. As scientists deepen their understanding, osmosis continues to illuminate pathways—bridging cells, tissues, and entire organisms in a unified story of balance, motion, and life itself.


Related Post

Osmosis Defined: The Silent Diffusion That Powers Life and Engineered Systems
Rachel’s Twisted Fate: What Really Happened in Ozark Season 2

André-Louis Auzière: Fuselor of Industrial Innovation Behind 19th Century Industrial Progress

Pete Sampras: The Serving Legend Who Redefined Tennis Dominance

