The Lewis Structure of SCN⁻: Decoding the Chemical Blueprint of a Versatile Ion
The Lewis Structure of SCN⁻: Decoding the Chemical Blueprint of a Versatile Ion
In the intricate world of inorganic chemistry, few species intrigue as deeply as the thiocyanate ion—SCN⁻. With a structure that belies its complex reactivity, SCN⁻ combines sulfur, nitrogen, and carbon through a single central atom, forming a versatile intermediate in chemical reactions, pharmaceuticals, and analytical techniques. Understanding its Lewis structure reveals not just its electron distribution, but also the hidden mechanisms that drive its myriad roles in science and medicine.
Atomic Arrangement and Bonding: The Core of SCN⁻
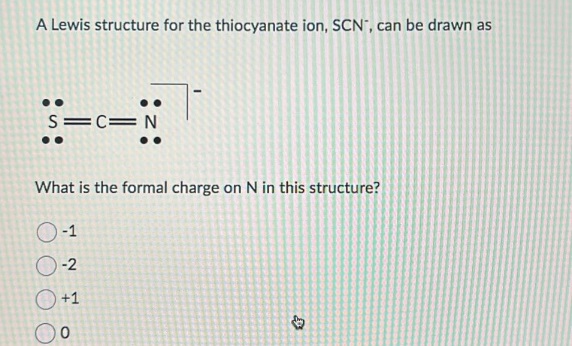
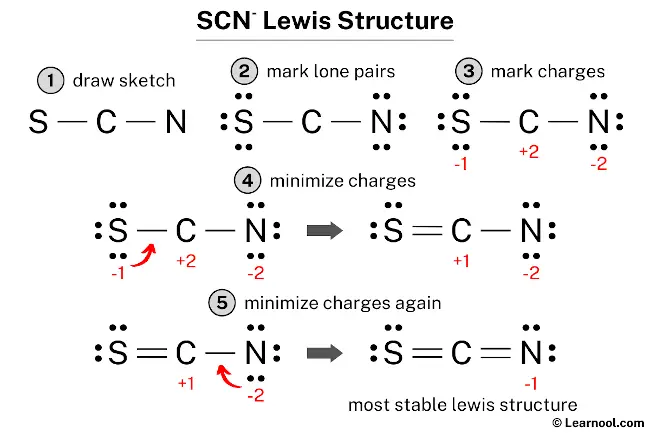
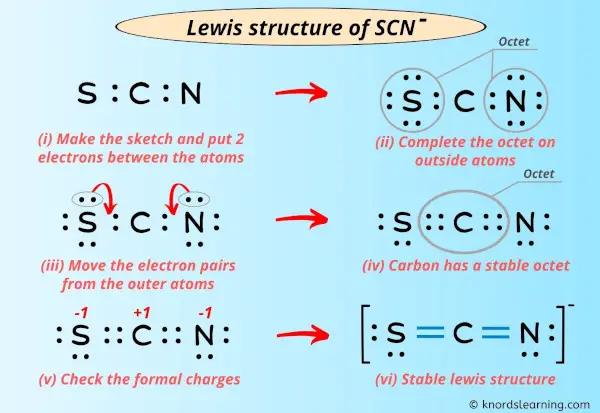
The Lewis structure for thiocyanate ion (SCN⁻) centers on sulfur, which serves as the central atom bonded to a sulfur-attached nitrogen and carbon, completing a resonance-stabilized anion with a net charge of –1. Sulfur, in Group 16, contributes six valence electrons—three unshared lone pairs and three shared in bonds. Nitrogen, from the nitrile triplet (C≡N), brings two bonding pairs through a single bond and two lone electrons.Carbon, typical of organic bonding, shares a single bond with nitrogen and another with sulfur, fulfilling the ion’s three-atom core. To sketch the Lewis structure, begin by placing sulfur at the center: S—N—C + two lone electrons on sulfur Each bond (S–N and S–C) is a covalent single bond, using four shared electrons. The lone pairs on nitrogen (two) and sulfur (three) balance the charge and satisfy octet rules via expanded sulfur valence—thorium and selenium can accommodate more than eight electrons, enabling sulfur to form three bonds and retain lone pairs.
Crucially, resonance stabilizes SCN⁻ by spreading negative charge across three positions: the lone pair on nitrogen can delocalize into the C≡N triple bond, shifting electron density equally among S–N, N–C, and S–C bonds. This resonance hybrid, depicted as SCN⁻ ↔ S═N⁻…C≡◄N⁻, underscores the ion’s dynamic nature.
Formal Charges and Structural Stability
Calculating formal charges clarifies SCN⁻’s thermodynamic favorability. For the major contributor (S≡N–C):Sulfur: 6 – (6 lone e⁻ + 3 /2 bonds) = 6 – (6 + 3) = –1
Nitrogen: 5 – (5 lone e⁻ + 1 bond) = 5 – (5 + 1) = –1
Carbon: 4 – (4 lone e⁻ + 1 bond) = 4 – (4 + 1) = –1 Though all formal charges are negative, the ion maintains neutrality overall—dominant charge resides on sulfur, but distributed electron density reduces localized instability.
This charge dispersion enhances solubility in water and reactivity in redox and nucleophilic reactions.
Despite these favorable properties, SCN⁻ remains structure-sensitive. Narrower bonds from dual nitrogen-carbon linkage and partial charge separation influence interactions with metals, enzymes, and other ions.
“The strength of SCN⁻ lies not just in its bonds, but in its ability to adapt,” notes Dr. Elena Martinez, inorganic chemist at MIT. This flexibility enables its dual affinity—binding transition metals while remaining reactive toward nucleophiles.
Resonance and Delocalization: The Hidden Dynamics
Resonance in SCN⁻ transforms it from a static ion into a shifting electron cloud. With both nitrogen and sulfur contributing lone pairs to double-bond character, electron density extends beyond localized covalent bonds. This delocalization lowers the ion’s energy and increases its stability in solution and crystalline phases.Key resonance forms include:
- SCN⁻ (neutral charge shift)
- S⁺–N≡C⁻
- S⁻–N≡C⁺
- S═N–C⁻ (partial double character)
Notably, in solid-state matrices, SCN⁻ often forms polydentate complexes, where its lone pairs bridge transition metals like iron, cobalt, and nickel in catalytic centers.
Such binding modes influence redox potentials and reaction pathways, making LS bonds critical in enzymatic catalysis and industrial applications.
Applications and Environmental Relevance
Beyond laboratory curiosity, SCN⁻ plays pivotal roles across disciplines. In biochemistry, it serves as a neurotransmitter precursor—global thiocyanate conjugates modulate cellular signaling and oxidative stress. Clinically, SCN⁻ detoxification pathways are leveraged in cyanide poisoning antidotes, where its displacement of oxygen in cytochrome c oxidase prevents cellular hypoxia.In analytical chemistry, SCN⁻ is a sensitive ligand for qualitative and quantitative metal detection—forms vivid turn-of-color complexes or fluorescent emissions distinguish metal ions in trace analysis. Its low toxicity and high affinity for heavy metals render it indispensable in environmental monitoring and wastewater treatment.
The Lewis Structure: A Gateway to Versatility
The Lewis structure of SCN⁻—though seemingly simple—unchains a nexus of chemical behavior.Resonance, charge delocalization, and structural flexibility converge to make this ion far more than a static anion. It exemplifies how molecular architecture dictates reactivity, stability, and function across scales—from biomolecules to industrial catalysts. Understanding its structure is not merely academic; it unlocks pathways to innovation in medicine, environmental science, and advanced materials.
What began as a discrete, linear sketch evolves into a dynamic portrait of chemical intelligence—proof that even the smallest ions shape the largest scientific frontiers.

Related Post

Columbia’s Most Successful Alumni: Architects of Global Influence

The Ultimate Guide to Exploring Global Cinema with Movies123 Go

Tw Trending Reveals How Smartphones Are Rewriting Daily Life—From Productivity to Mental Health

Map Location Of Siberia: The Vast, Enigmatic Heart of Russia

