Understanding Glioblastoma: The Deadly Graduate of Brain Tumors
Understanding Glioblastoma: The Deadly Graduate of Brain Tumors
Glioblastoma, classified as an astrocytoma Grade 4, stands as the most aggressive and common primary malignant brain tumor in adults, posing unprecedented challenges to medicine and patients alike. With a five-year survival rate hovering around 6–10%, it remains the deadliest form of cancer originating in the brain, combining relentless growth, invasive behavior, and resistance to both surgery and therapy. Unlike lower-grade astrocytomas, Glioblastoma’s extreme aggressiveness stems from its ability to infiltrate surrounding neural tissue, forming a chaotic, poorly defined mass that defies complete surgical removal and rapidly undermines quality of life.
Unique among brain tumors, Glioblastoma’s microscopic profile reveals a complex cellular makeup dominated by rapidly dividing glial cells—specifically astrocytes—exhibiting hallmark features of uncontrolled proliferation and genomic instability. These tumors are defined clinically by the World Health Organisation’s Grade 4 criteria, reflecting their pronounced mitotic activity, microvascular proliferation, and necrotic centers. “Glioblastoma isn’t just a tumor—it’s a biological storm,” says Dr.
Elena Torres, neuro-oncologist at the National Cancer Institute. “It grows swiftly, metastasizes silently, and adapts faster than current treatments can respond.”
At the cellular level, Glioblastoma arises from astrocytes—star-shaped support cells critical to brain homeostasis. While the exact origin remains partially elusive, emerging research suggests that mutations in key genes such as IDH1, TP53, and EGFR drive oncogenic transformation, fueling unchecked cell division and evasion of apoptosis.
The loss of tumor suppressor function, particularly in genes regulating DNA repair and cell cycle checkpoints, enables the accumulation of genetic chaos that defines this high-grade malignancy. “What makes Glioblastoma uniquely destructive is its ability to rewire the tumor microenvironment,” notes Dr. Raj Patel, a leading researcher in neuro-oncology.
“It co-opts blood vessels, suppresses immune surveillance, and creates a hostile milieu that shields cancer cells while starving surrounding tissue.” The clinical impact of Glioblastoma is defined by its rapid progression and invasive nature. Patients typically endure worsening headaches, seizures, cognitive decline, and neurological deficits within months of diagnosis—symptoms driven by both mass effect and elevated intracranial pressure. Surgical resection remains the cornerstone of initial treatment, yet even gross total resection leaves behind microscopic disease, as the tumor’s tendril-like extensions penetrate deep into functional brain regions.
“The brain is not a compartment—it’s beautifully interconnected,” explains Dr. Torres. “Even trained surgeons cannot distinguish tumor from healthy tissue at the cellular level, so residual cells often persist, reigniting the cancer days or weeks later.” Radiological imaging reveals Glioblastomas as illustration of their destructive potential: heterogeneous lesions with necrotic cores surrounded by enhancing margins, the latter reflecting aggressive vascular proliferation typical of high-grade gliomas.
Advanced diagnostic tools like magnetic resonance imaging (MRI) with contrast enhancement and perfusion mapping guide treatment plans, but no imaging modality fully captures the tumor’s microscopic invasiveness. “Diffusion tensor imaging helps chart extent, yet subtle infiltration beneath the blood-brain barrier often escapes detection,” warns Dr. Patel.
“This hidden spread makes complete eradication nearly impossible.” Therapeutic strategies hinge on a triad of surgery, radiation, and chemotherapy, with temozolomide—the standard chemotherapeutic agent—delivered intravenously following surgery to cross the blood-brain barrier and attack rapidly dividing cells. Radiation therapy, typically delivered in fractionated doses over six weeks, aims to target residual tumor cells while managing toxicity to adjacent neural structures. Yet resistance remains a critical hurdle: Glioblastoma’s genetic plasticity enables adaptive resistance, such as upregulation of DNA repair pathways or activation of alternative survival signaling.
“Tumors evolve fast—within months, new mutation clones emerge that defy therapy,” notes Dr. Torres. “This is why a single treatment rarely lasts.” Emerging therapies seek to disrupt the tumor’s complex ecosystem.
Immunotherapies, including checkpoint inhibitors and personalized neoantigen vaccines, aim to awaken the immune system, though the tumor’s immunosuppressive microenvironment limits response rates. Concurrently, targeted agents under clinical investigation block key oncogenic drivers—such as EGFR inhibitors—and disrupt the chaotic vascular network enabling tumor growth. “We’re moving beyond one-size-fits-all approaches,” says Dr.
Patel. “Precision medicine, combining genomic profiling with novel drug combinations, offers our best hope for extending survival.” The future of Glioblastoma research is anchored in early detection and dynamic monitoring. Liquid biopsies analyzing cerebrospinal fluid or blood for tumor DNA variants promise non-invasive tools for tracking disease burden and treatment response.
Wearable sensors and cognitive assessments may detect subtle functional declines before they become clinical crises. “The key is not just to slow growth, but to prevent recurrence through constant, proactive monitoring,” explains Dr. Torres.
“Glioblastoma is not static—it’s a moving target.” Despite decades of research, Glioblastoma remains one of oncology’s most formidable adversaries, challenging medical science with its complexity, resilience, and impact. While radical cures remain elusive, advances in understanding its biology and treatment have gradually shifted the boundaries of what’s possible. Every breakthrough, from identifying key driver mutations to refining immunotherapeutic strategies, brings hope—not just for longer survival, but for a future where this deadly tumor is no longer a death sentence, but a manageable disease.
The journey continues, relentless and deeply human, as researchers and clinicians push the limits of innovation to outsmart cancer’s most aggressive form.

.jpg)


Related Post

Z-Library on iPad: The Ultimate Guide to Download and Use E-Books Effortlessly
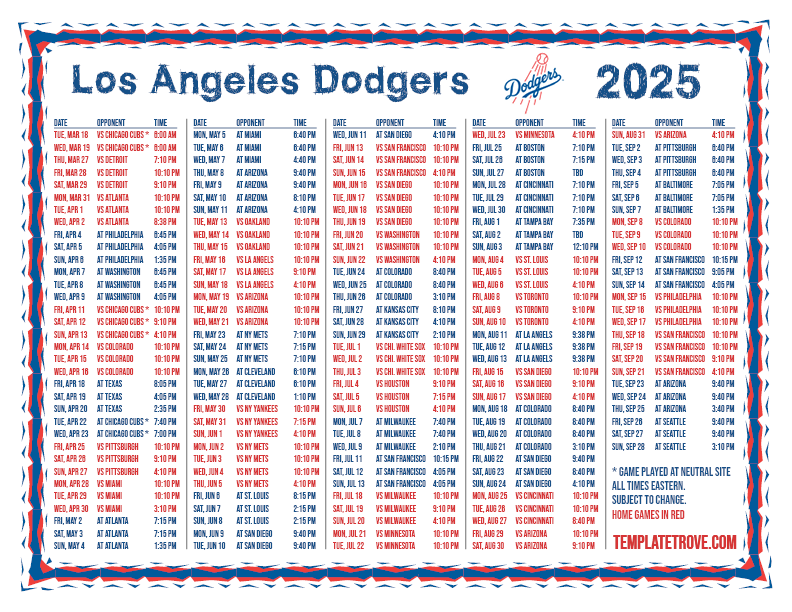
2025 Dodgers Home Game Schedule & Essential Guide: Everything Fans Need to Know to Own June to August on the Field
Fresno CA Zip Codes Your Quick Guide: Navigate the Valley by Postal Precision

Is Matthew Beard Married? The Untold Truth About His Personal Life

