Unlocking Atomic Forces: How Coulombic Attraction Powers the Foundation of Matter
Unlocking Atomic Forces: How Coulombic Attraction Powers the Foundation of Matter
At the heart of chemistry and physics lies an invisible yet unyielding force—Coulombic attraction—the fundamental electrostatic pull that governs how atoms bond, molecules form, and matter stabilizes. This Coulombic dance of positive and negative charges governs the behavior of subatomic particles and serves as the cornerstone of chemical reactivity and molecular structure. Through the structured approach of the Pogil Active Learning Program, students grasp this core concept not just as abstraction, but as a tangible, observable force driving the real-world phenomena that shape life itself.
Understanding Coulombic attraction requires deep insight into the interaction between charged particles—how protons and electrons engage across distances, guided by Coulomb’s Law. This law, formulated by Charles-Augustin de Coulomb in the 18th century, quantifies the electrostatic force between two point charges: it states that the force increases with charge magnitude and decreases with the square of the distance separating them. “The force is directly proportional to the product of the charges and inversely proportional to the square of the distance,” accurately capturing how even subtle shifts in charge or spacing dramatically alter attraction strength.
Within the Coulombic Attraction module promoted by Pogil, learners are guided step-by-step through the principles behind this force. The program emphasizes active engagement—students analyze atomic configurations, calculate repulsive and attractive forces, and predict molecular behavior based on charge interactions. “Coulombic forces determine electron arrangement, molecular polarity, and ultimately, the physical properties of substances,” explains a core Pogil lesson.
This hands-on exploration demystifies abstract concepts by linking theory directly to real-world outcomes such as hydrogen bonding, ionic lattice stability, and covalent bond directionality.
Central to mastering Coulombic attraction is recognizing the role of charge magnitude and distance. Positive and negative charges attract; like charges repel.
The balance between these forces dictates electron density, polarity, and interaction energetics. For example:
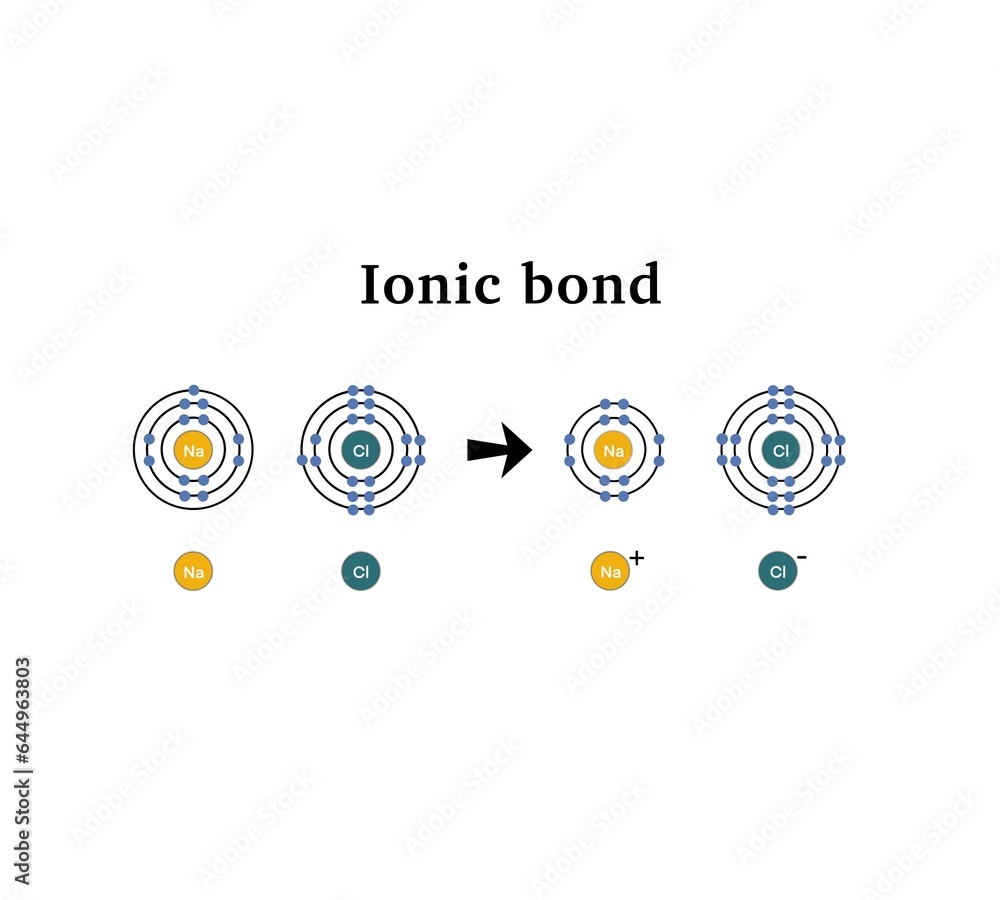
- Atoms in Ionic Bonds: When sodium (Na⁺) transfers an electron to chlorine (Cl⁻), Coulombic attraction stabilizes the resulting ionic lattice. The strong electrostatic draw between oppositely charged ions lowers system energy and fixes structure.
- Water’s Hydrogen Bonding: Though not a classical Coulombic ionic interaction, hydrogen bonds between water molecules rely on partial charges and attraction, emerging from electronegativity differences and electrostatic pull—consistent with Coulombic principles.
- Molecular Geometry: Electron pair repulsion, explained through VSEPR theory, stems from Coulombic repulsions between negatively charged electron domains, shaping bond angles and 3D architecture.
Key variables in Coulombic attraction include charge magnitude, electronegativity, and interatomic distance.
These shape: \begin{itemize} \item Bond strength and length \item Ionization energies \item Polarity and solubility \item Acid-base behavior and proton affinity \end{itemize> For instance, fluorine’s high electronegativity creates strong Coulombic attraction to hydrogen in HF, producing a polar covalent bond—sharpening perspectives on intermolecular forces and reactivity.
The Pogil curriculum ensures that these relationships aren’t taught in isolation but woven into rich, contextual learning. Active reading, collaborative problem-solving, and conceptual mapping foster deep retention.
Students don’t just memorize Coulomb’s Law—they apply it to predict why noble gases seldom bond, why metallic bonds rely on delocalized electrons experiencing constant Coulombic attraction to surrounding ions, and how biologists and chemists design drugs by modeling molecular docking via charge interactions.
Beyond the classroom, Coulombic attraction underpins innovations in materials science, nanotechnology, and drug discovery. Programmable materials with tunable electrostatic properties emerge from precise manipulation of charge distributions.
“This force is not just lab-bound—it’s quantum in nature yet macroscopically decisive,” notes a physics education researcher cited in recent studies. “Understanding Coulombic attraction enables engineers to design stronger, smarter, and more adaptive systems.”
In essence, the Coulombic Attraction module, as taught through Pogil’s five-step active learning framework, transforms an abstract force into a lived scientific principle—one students can visualize, compute, and apply. The invisible attraction between charged particles reveals the hidden architecture of matter, connecting microscopic interactions to the observable world in a way that is both rigorous and profoundly captivating.
As learners progress, they see Coulomb’s insight not as a relic of classical physics, but as the enduring foundation upon which modern chemistry, biology, and technology are built.
Mastery of Coulombic attraction equips students with a lens to decode nature’s molecular logic—one that remains indispensable across scientific disciplines. From isotopic bonding to pharmaceutical innovation, this force remains Scotland’s own silent architect: shaping atoms, binding molecules, and driving progress through invisible yet unbreakable electrostatic harmony.




Related Post

The Fundamental Dance of Opposites: Coulombic Attraction in Electrostatic Interactions
Mastering Coulombic Attraction: The Core of Electrostatic Forces in Chemistry

Coulombic Attraction: The Invisible Force Shaping Atomic Structure—Pogil Answers that Illuminate the Basics

Sean Penn’s Health Journey: courage, chaos, and the unflinching reality of living with illness

