Unlocking Life’s Power: How the Cellular Respiration Equation Fuels Every Breath
Unlocking Life’s Power: How the Cellular Respiration Equation Fuels Every Breath
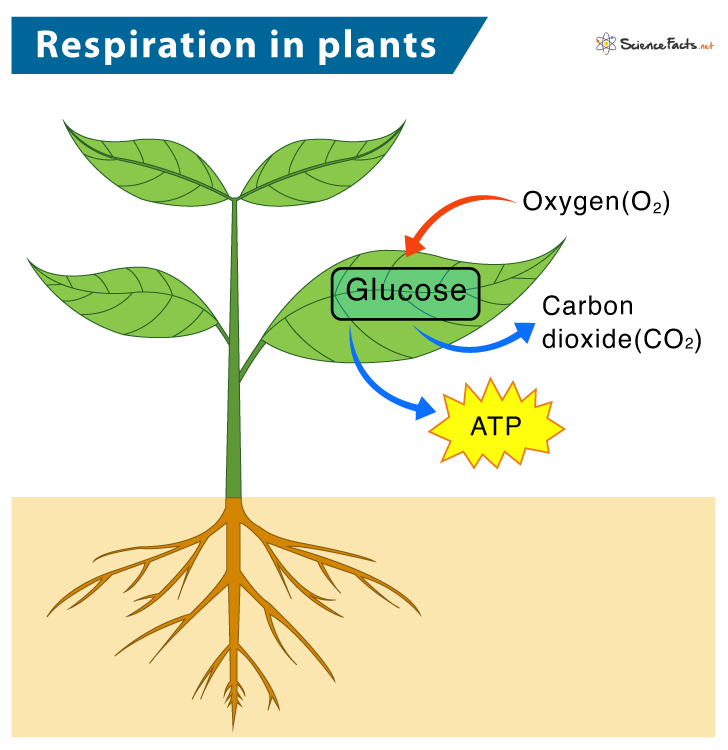
At the core of every living cell lies a silent, powerful engine: cellular respiration. This biochemical process transforms nutrients into usable energy, enabling life-sustaining functions across organisms. Central to understanding this process is the molecular equation that encapsulates it — C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + energy (ATP).
This simple yet profound equation reveals how glucose and oxygen unite to generate adenosine triphosphate (ATP), the energy currency driving cells from microbes to humans. With approximately 36 to 38 ATP molecules produced per glucose molecule, this chemical reaction powers everything from muscle contraction to neural signaling and biosynthesis.
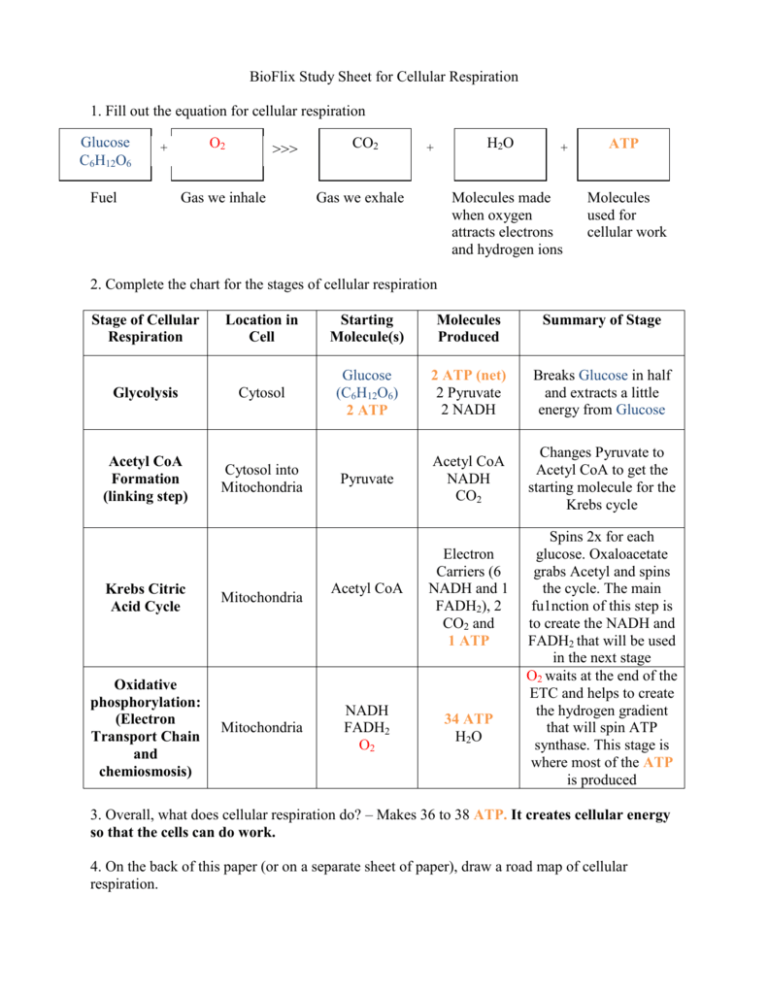
Cellular respiration comprises three primary stages — glycolysis, the Krebs cycle, and the electron transport chain — each intricately spaced to maximize energy extraction.
Glycolysis begins in the cytoplasm, where one glucose molecule is split into two pyruvate units, yielding a modest 2 ATP and 2 NADH. “Glycolysis is the metabolic gateway that prepares glucose for deeper energy release,” explains Dr. Elena Torres, a biochemist at MIT.
“Though limited in yield, it launches the cascade by generating key electron carriers.” As pyruvate enters the mitochondria, it is converted into acetyl-CoA, feeding into the Krebs cycle where carbon skeletons are processed, releasing CO₂ and transferring high-energy electrons via NADH and FADH₂. The real energy concentration stage unfolds in the inner mitochondrial membrane, where the electron transport chain orchestrates a proton gradient, culminating in ATP synthase-driven ATP production — the highest yield of the process.
The full cellular respiration equation — C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + ~32 ATP (varies by cell type)—is more than a Chemical formula.
It is a blueprint of life’s energy flow. Each reactant and product reflects biological precision: glucose serves as the primary fuel, molecular oxygen acts as the final electron acceptor, and water represents both a by-product and a life-sustaining molecule. Carbon dioxide leaves the cell,차지ing respiratory exchange and signaling metabolic status to the environment.
Meanwhile, ATP is shuttled to sites of need — mitochondria in muscle, neurons, kidneys — proving the equation’s real-world utility beyond textbooks.
Understanding this process illuminates fundamental truths about health, evolution, and biotechnology. “Cells across life have conserved this pathway because it’s efficient, scalable, and adaptable,” says Dr.
James Wu, a cellular biologist at Stanford. “Whether in yeast fermenting bread or human hearts sustaining life, the equation remains central.” Beyond physiology, researchers manipulate cellular respiration for medical therapies, biofuel development, and synthetic biology, highlighting its transformative role beyond basic biology.
From the microscopic dance of enzymes to the macroscopic limits of endurance, cellular respiration powers existence.
The equation captures not just chemistry, but the very rhythm of life — where fuel burns, energy surges, and life endures. As scientists continue decoding its nuances, one certainty remains: this simple equation is the heartbeat of biology, written in every breath, heartbeat, and metabolic breath.
The Pulse of Energy: Breaking Down the Cells’ Powerhouse Equation
The cellular respiration equation — C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + ATP — is the molecular cornerstone of energy production, central to nearly all life forms. At its heart, this reaction encapsulates a stepwise breakdown of glucose to yield adenosine triphosphate (ATP), the universal energy carrier.Each component of this equation reflects a precise biological transformation: glucose, a six-carbon sugar, is oxidized in a series of high-complexity reactions, while oxygen serves as the final electron acceptor, forming water. The energy released drives proton pumps across mitochondrial membranes, enabling local electrochemical gradients that power ATP synthase, the enzyme responsible for generating most ATP molecules.
Glycolysis initiates this cascade in the cytoplasm, splitting glucose into two molecules of pyruvate, producing a net of 2 ATP and 2 NADH.
The equation’s entry into the mitochondria marks a pivotal shift: pyruvate is converted to acetyl-CoA, linking anaerobic and aerobic processes. Within the Krebs cycle, each acetyl unit fuels a series of redox reactions yielding NADH, FADH₂, and GTP — coupled with CO₂ efflux — that feed electrons into the electron transport chain. “This chain is a highly organized molecular machine,” notes Dr.
Torres. “It translates electron energy into proton motive force, an electrochemical gradient used to drive ATP synthesis.” Ultimately, oxygen’s role is indispensable: as the terminal electron acceptor, it enables the chain to function efficiently, making water both a waste product and a critical rescuer of atmospheric chemistry.
The stoichiometry of the equation reflects biological economy: 1 mole of glucose, paired with 6 moles of oxygen, produces up to 30–32 ATP molecules depending on cell type and aerobic conditions.
“Each ATP synthesized represents stored potential energy,” explains Dr. Wu. “Clearly, this equation does more than describe chemistry — it tells the story of how organisms capture and harness energy efficiently.” Variations in ATP yield across tissues and organisms underscore the adaptability of cellular respiration, yet the core equation remains invariant — a tribute to evolutionary precision.
Beyond its biochemical elegance, the equation drives innovations in medicine, energy, and environmental science. Understanding how cells use oxygen and glucose informs treatments for metabolic disorders and respiratory diseases. In synthetic biology, scientists engineer microbes to optimize this pathway for sustainable biofuel production.
Even tumor cells rewire their respiration — a phenomenon known as the Warburg effect — showcasing how the equation adapts to pathological states.
In sum, cellular respiration is far more than a chemical equation. It is the engine of life, turning molecular fuel into vitality with remarkable efficiency and elegance.
The equation C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + ATP is both a scientific marvel and a biological necessity — the pulse that sustains every living cell, from microscopic single-celled organisms to the human body. As research advances, this equation continues to illuminate the intricate, energy-driven dance of life itself.


Related Post

Jeri Ryan’s Rise to Stardom and Her Enduring Financial Legacy: Who Is the Renowned Star Behind the Number?
.jpeg?quality=75&width=1200&auto=webp)
Where Does Elon Live? The Lavish Styles and Secrets of Tesla and SpaceX CEO’s Residential Haven

What Zodiac Sign Governs June 14th? The Truth Behind Your Cosmic Identity

Exploring Rosario Dawson’s Marriage: A Deep Dive into Romance, Resilience, and Shared Purpose

