Unlocking the Chemistry of Potassium: Decoding Its Essence via Lewis Dot Structure
Unlocking the Chemistry of Potassium: Decoding Its Essence via Lewis Dot Structure
Lewis Dot Structures for potassium (K) reveal a fundamental insight into its atomic behavior—how this highly reactive alkali metal shares electrons in ways that define its chemical identity. As one of the most abundant elements on Earth, potassium’s unique properties hinge on a simple yet revealing electron arrangement, visualized through dots and lines that map its valence electrons and bonding tendencies. Understanding these structures is pivotal, not only in inorganic chemistry but in fields ranging from biology to materials science.
At the heart of chemical reactivity lies electron configuration, and potassium’s position in Group 1 of the periodic table underscores its penchant for electron loss. With a single valence electron in its outermost shell, potassium readily satisfies the octet rule by shedding this electron to achieve stability—a hallmark of alkali metal behavior. The Lewis dot structure captures this core mechanism: a central potassium symbol surrounded by a pair of dots, representing its lone valence electron.
In K, the symbolic representation—a single K with three dots surrounding it—visually conveys both electron count and the drive toward chemical stability.
Decoding the Lewis Dot Structure for Potassium
The Lewis dot structure for potassium simplifies a complex quantum reality into a clear, symbolic blueprint. Each dot indicates a single valence electron; in potassium’s case, only one is shown, emphasizing its extreme reactivity. This minimal count contrasts sharply with elements seeking stable electron configurations, such as noble gases.The structure may also include a notation for ionization, especially in compound formation: when potassium donates its electron during ionic bonding, it becomes the K+Structural Simplicity: Unlike molecules with complex bonding patterns, the potassium dot structure features only one atom, limiting its Lewis depiction to a single central dot around K. - Electron Dynamics: The solitary dot underscores potassium’s role as a powerful reducing agent—capable of easily transferring its electron to electron-deficient species.
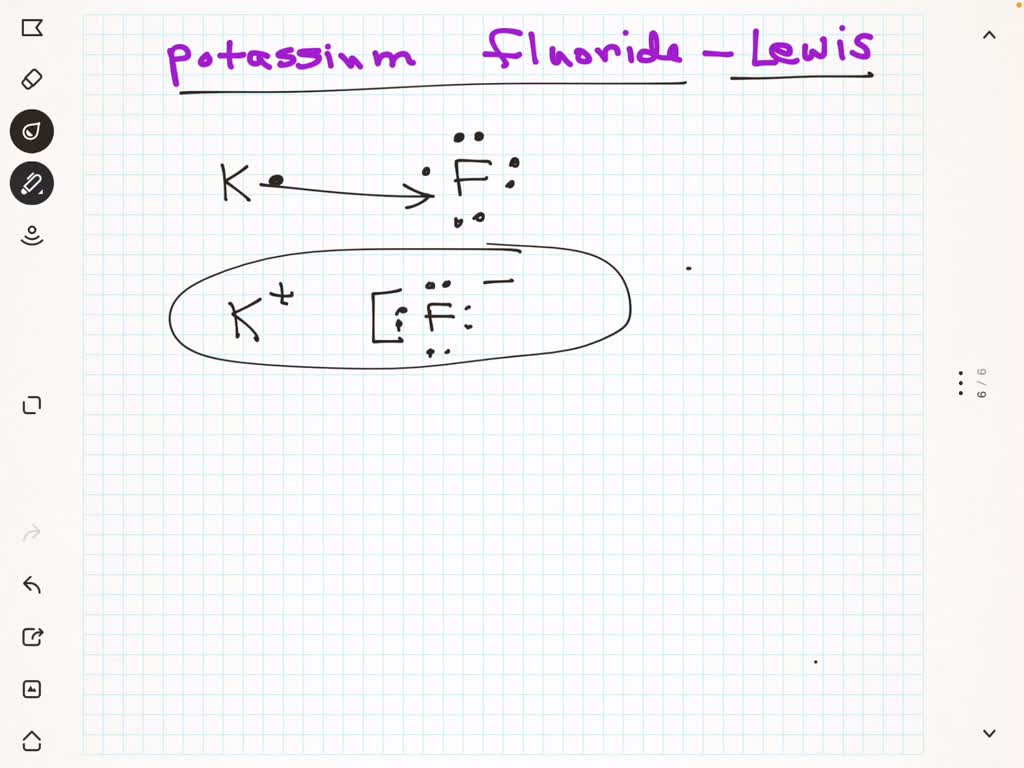
- Formation of Ions: When bonding, the dot structure evolves to reflect charge; K in K+The formation of potassium chloride (KCl) exemplifies this electron transfer. In this compound, potassium donates its valence electron to chlorine—a highly electronegative element unable to share electrons affordably.
The dot structure reflects this exchange: K appears with three dots, while Cl (symbolized ‘Cl’) is completed with seven dots (three from lone pairs, four implied via formal charge or extended representation) to mirror its octet. This shift from K• to K+– illustrates how Lewis notation captures bonding beyond mere atoms, illustrating charge formation and ionic stoichiometry.
The Significance of Electron Configuration
Potassium’s chemical identity is inseparable from its simplified electron layout. With just one valence electron in the 4s orbital, potassium aligns with Group 1’s predictable behavior—eager to lose one electron and form a +1 ion.This dynamic is not merely theoretical; it drives potassium’s extensive use in living systems and industrial applications. Biologically, K+3 (nitric acid salt) and K2SO4 (potassium sulfate) are foundational in fertilizers and chemical synthesis, their reactivity rooted in potassium’s electron-sharing propensity.
What makes potassium’s Lewis structure particularly instructive is its transparency. It strips away the complexity of orbitals and wave functions to highlight only what matters: electron count, charge, and bonding potential. This clarity allows chemists to predict reactivity, design compounds, and understand periodic trends with precision.
As one inorganic chemistry manual notes, “The Lewis dot structure of alkali metals like potassium is not just a drawing—it is a gateway to anticipating how elements will behave when immersed in chemical environments.”
Visualizing Bonding and Molecular Formation
While potassium rarely forms discrete molecules due to its strong metallic bonds and ionic tendencies, understanding its dot structure illuminates how it integrates with other atoms. In ionic salts, K+2O, chlorine in KCl—forming extended lattices stabilized by electrostatic forces. The dot structure evolves to reflect these extended interactions, even if each bond leaf implies a localized dipole formatted by electronegativity differences.Thermal and electromagnetic properties similarly trace back to this atomic-level behavior. Potassium’s low ionization energy—evident in its quick loss of the valence electron—explains its high reactivity with water, forming KOH and releasing hydrogen gas. The single dot acts as a visual proxy for energy thresholds, where even minimal excitation enables electron release and exothermic reaction.
In solid-state applications, potassium’s metallic nature—driven by its free



Related Post

Fox Star Studios: The Architect of Indian Entertainment’s Golden Era

Jackson Hole’s Live Music Scene: Where Mountain Echoes Meet Melody

Ackermans Cell Phone Deals: Your Guide To Savings

MonografASobreLaContaminaciNAmbiental: El Impacto Silencioso del Amanecer de la Polución Urbana y Rural

