Vesicles: The Silent Messengers of the Animal Cell
Vesicles: The Silent Messengers of the Animal Cell
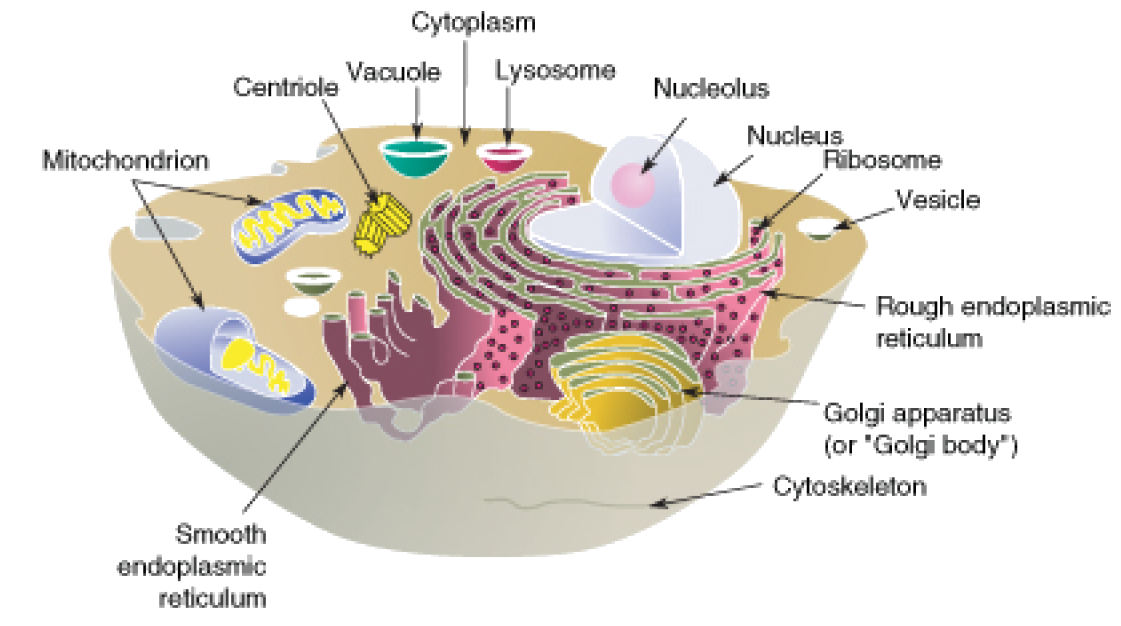
Ultrafine, membrane-bound sacs that shuttle proteins, fats, and signaling molecules within animal cells, vesicles are indispensable to cellular function. These nanoscale carriers operate behind the scenes, orchestrating essential processes such as protein secretion, nutrient delivery, and intracellular signaling. Far more than passive containers, vesicles actively regulate trafficking pathways that maintain tissue integrity and enable responsive communication.
From the secretory pathway to endocytic recycling, vesicle dynamics define how animal cells maintain homeostasis and interact with their environment.
Types and Roles of Vesicles in Cellular Transport
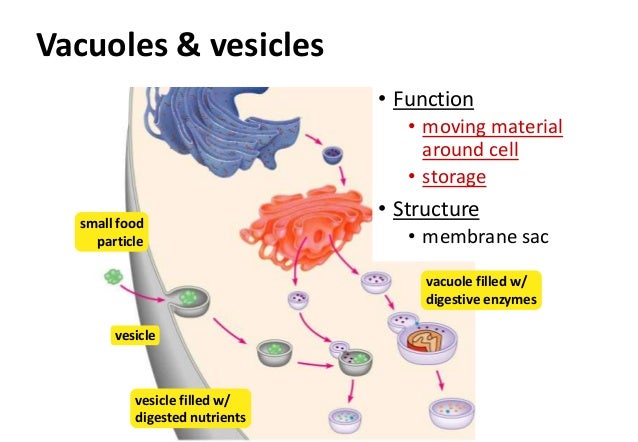
Not all vesicles are created equal—each serves a specialized function governed by its membrane composition and target destination. - **Transport vesicles**: These short-lived containers shuttle materials between organelles. For example, ER-to-Golgi vesicles carry newly synthesized proteins, while Golgi-to-plasma membrane vesicles deliver lipids and glycoproteins for secretion.
- **Lysosomal vesicles**: Integral to cellular cleanup, these vesicles fuse with acidic lysosomes to degrade damaged organelles, misfolded proteins, and pathogens. As biochemist Dr. Elena S.
Carter notes, “Lysosomal vesicles act as molecular recyclers, ensuring cellular turnover remains efficient and contamination-free.” - **Endocytic vesicles**: Formed when the plasma membrane invaginates to internalize extracellular materials, these vesicles deliver nutrients, hormones, and immune signals. Clathrin-coated vesicles, for instance, mediate receptor internalization after ligand binding, enabling essential signaling cascades. - **Secretory vesicles**: Accumulate cargo in preparation for exocytosis, these mature vesicles fuse with the plasma membrane to release their contents into the extracellular space—critical in neurons, endocrine cells, and immune cells.
Each vesicle type operates with precision, guided by molecular motors, Rab GTPases, and SNARE proteins that ensure correct targeting and timing.
Vesicle Biogenesis: From Golgi Pivot Point to Targeted Delivery
The Golgi apparatus serves as the central hub for vesicle formation and sorting. Newly synthesized proteins from the endoplasmic reticulum (ER) are packaged into COPII-coated vesicles for transport to the Golgi.
Once at the Golgi, vesicles inherit unique cargo markers that dictate their final destination. - Vesicles budding from the **cis-Golgi** deliver lipids and secretory proteins to the plasma membrane. - Those emerging near the **trans-Golgi network** target lysosomes, endosomes, or secretory sites.
- Retrieval vesicles, formed at the plasma membrane, recycle receptors and lipids back to the Golgi and ER. This dynamic sorting process relies on specific Golgi enzymes and membrane proteins that recognize sorting signals. “The Golgi is not just a static station,” explains cell biologist Dr.
Marco Lin. “It’s a decision-making center where vesicle identity is refined at every step.” Venues of Biogenesis: Cytosome vs. Exosome Pathways
While most vesicles originate in the Golgi, some form through alternative routes.
Under stress or specific cellular conditions, fragments of endosomal membranes bud into multivesicular bodies (MVBs), generating intraluminal vesicles that mature into exosomes—small vesicles 30–150 nm in diameter. These exosomes carry microRNA, antigens, and signaling molecules, playing key roles in immune modulation and intercellular communication.
> Unlike classical transport vesicles, exosomes are released upon MVB fusion with the plasma membrane, enabling cells to “send secret messages” without direct contact. Research from Harvard’s Meanwhile, Hu’s lab highlights exosomes as “biological 기업 espionage tools” influencing neighboring and distant cell behavior—from tumor signaling to neural synapse remodeling.
Regulation and Surveillance: Ensuring Vesicle Accuracy
Propagating errors in vesicle transport can disrupt cellular harmony. To prevent trafficking mistakes, cells employ rigorous quality control mechanisms. - Rab GTPases act as molecular switches, directing vesicles along specific routes by binding GSTM7 or RAB proteins.
- SNARE proteins mediate fusion specificity: v-SNAREs on vesicles link with t-SNAREs on target membranes, preventing wrong-wrong docking. - Ubiquitin tags route misfolded proteins to lysosomes for degradation, safeguarding protein integrity. Disruptions in vesicle regulation can lead to severe pathologies, including neurodegenerative diseases like Alzheimer’s and lysosomal storage disorders, where inefficient clearance results in toxic buildup.
Implications for Health and Disease
Vesicle function is a linchpin in both health and disease. In diabetes, defective insulin vesicle trafficking impairs secretion in pancreatic beta cells, contributing to insulin deficiency. Similarly, mutations in lysosomal vesicle enzymes cause rare storage diseases such as Tay-Sachs and Gaucher’s, marked by toxic metabolite accumulation.
Emerging research highlights vesicles as therapeutic targets. Exosome engineering, for instance, shows promise in delivering drugs precisely to cancer cells or repairing damaged neural tissues. Eric Kool’s team at Stanford demonstrates how manipulating vesicle cargo can enhance targeted drug delivery, reducing off-site side effects.
Vesicles Beyond Transport: Signaling and Tissue Coordination
Vesicles increasingly recognized as active participants in cellular signaling. Their lipid bilayers and surface receptors enable direct intercellular communication, linking metabolic states across tissues. In immune systems, antigen-presenting vesicles educate T cells, shaping adaptive responses.
In neurons, regulated vesicle exocytosis transmits neurotransmitters across synapses. This functional versatility positions vesicles not just as logistical tools, but as vital communicators in multicellular networks.
The story of vesicles in the animal cell is one of complexity, precision, and adaptability.
These microscopic carriers are orchestrators of life at the cellular level, aligning intracellular processes with external demands. As research unfolds, their role continues to expand—from secretory hubs to conversation starters between cells. Vesicles are proving indispensable pillars of cellular intelligence, underpinning both routine operation and responses that shape health and disease.
Related Post

Gabriel Swaggart: Age, Wife, and the High-Stakes Crossfire of Faith, Love, and Public Scrutiny

What Does It Mean to “Understand” in the Age of Information Overload?

Aitana Bonmatí’s Relationship with Her Boyfriend: A Story of Quiet Strength and Shared Dreams

Cricket Wireless 5G Home Internet: Your Complete Guide to Ultra-Fast, Reliable Wireless Connectivity

