What Is Electronic Spectra? Unlocking Matter Through Light’s Fingerprint
What Is Electronic Spectra? Unlocking Matter Through Light’s Fingerprint
Electricity shapes the invisible fabric of modern science, but few realize that its true power lies in what it reveals when matter interacts with electromagnetic radiation—this is the essence of electronic spectra. Electronic spectra refer to the detailed patterns generated when electrons in atoms or molecules absorb or emit light across specific wavelengths, producing a unique signature that identifies chemical composition, bonding states, and energy transitions. Far more than mere visual data, these spectra serve as a molecular fingerprint, enabling scientists to decode the unseen world of quantum mechanics and chemical interactions with remarkable precision.
At the heart of electronic spectra is the quantum behavior of electrons. In atoms, electrons occupy discrete energy levels, much like steps on a staircase. When a photon—particle of light—with energy matching the difference between two electron levels strikes an atom, it can be absorbed, promoting the electron to a higher energy state.
This process, known as electronic transition, results in a distinct absorption peak in the spectrum. Conversely, when an excited electron returns to a lower level, it releases energy as a photon, producing an emission peak. Electronic spectroscopy captures these absorption and emission patterns across ultraviolet (UV), visible (Vis), and sometimes infrared (IR) regions of the electromagnetic spectrum.
Each element and compound generates a characteristic set of spectral lines, akin to a musical note sequence, allowing researchers to identify substances down to parts-per-million concentrations. For example, the sharp blue lines in the emission spectrum of hydrogen—Balmer series—reveal its presence in stars and nebulae. Similarly, the broad absorption bands in a dye molecule’s UV-Vis spectrum expose conjugated double-bond systems critical to light absorption in solar cells.
How Electronic Spectra Reveal Atomic and Molecular Identity
The unique nature of electronic spectra stems from the quantized energy states governed by quantum mechanics. Unlike continuous energy distributions expected in classical physics, electrons in bound systems exist only in certain allowed states, explained by the Schrödinger equation. Transitions between these states follow strict selection rules, influencing which wavelengths are absorbed or emitted.For instance, in multi-electron atoms, electron-electron repulsion distorts energy levels, causing splitting and shifting that produce complex but interpretable spectral fingerprints. Take the case of transition metal ions—renowned for their colorful compound colors. Chromium(III) ions in sapphire produce vibrant blue hues entirely due to electronic transitions in the visible range, absorbed at specific wavelengths and reflected as blue light.
Such transitions arise from d-orbital splitting in crystal fields, a phenomenon directly observable through electronic spectra. Beyond elemental fingerprints, electronic spectra decode molecular structure. Conjugated organic molecules like β-carotene exhibit extended π-electron systems, resulting in low-energy transitions readily probed by UV-Vis spectroscopy.
The position, width, and intensity of absorption bands inform chemists about bond lengths, angles, and electron delocalization. In advanced applications, fluorescence spectroscopy enhances sensitivity by measuring emitted light after excitation, enabling real-time tracking of dynamic processes in chemistry and biology. Analyzing electronic spectra relies on spectrometers—precision instruments that disperse light by wavelength.
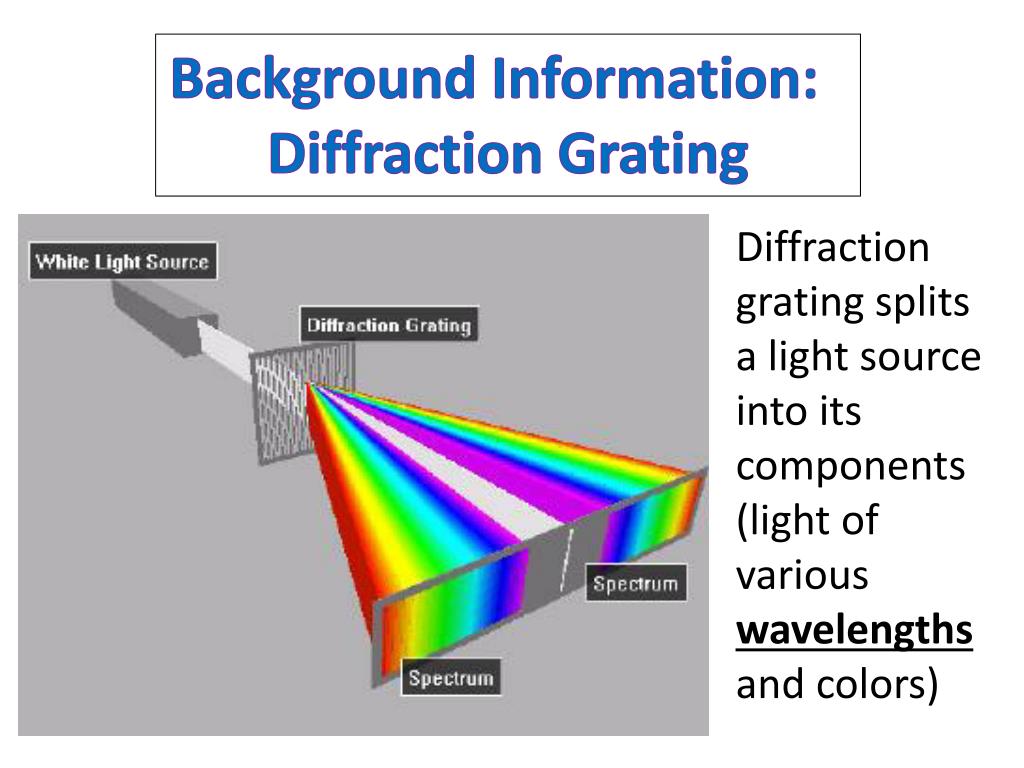
A typical setup includes a light source, sample holder, dispersive element (diffraction grating or prism), and detector. The resulting spectrum, displayed as intensity versus wavelength, allows precise fitting of peaks to theoretical models. Sophisticated software performs curve fitting and peak deconvolution, even in complex mixtures, by matching observed patterns to known reference spectra in extensive databases.
Applications span astronomy, environmental science, and materials engineering. Astronomers analyze stellar spectra to determine stellar composition, temperature, and motion—each spectral line a clue to cosmic origins. In environmental monitoring, UV-Vis spectroscopy detects pollutants like nitrates or organic contaminants in water with high accuracy.
Meanwhile, photovoltaic research leverages electronic spectra to optimize semiconductor bandgaps, improving solar cell efficiency. Despite technological advances, challenges remain. Spectral overlap in complex mixtures requires innovative deconvolution techniques.
Noise and instrument calibration affect data fidelity, demanding rigorous protocols. Emerging optical technologies, such as laser-induced breakdown spectroscopy and ultrafast laser spectroscopy, promise higher resolution and real-time analysis, expanding domain boundaries. In scientific inquiry, electronic spectra are not just data points—they are stories written in electromagnetic language.
From identifying ancient pigments in Renaissance paintings to detecting biomarkers in living tissue, these spectra translate invisible light into profound biological and physical insight. The process remains rooted in fundamental quantum principles, yet its impact resonates across disciplines, bridging the microscopic and macroscopic worlds. As analytical tools evolve, electronic spectra will continue to illuminate the unseen, transforming how we understand the material universe.
Electronic spectra represent a cornerstone of modern analytical science, offering a window into the quantum world through the universal language of light. Their precision, versatility, and depth of information make them indispensable in both fundamental research and applied innovation, embedding them deeply into the fabric of technological progress.



Related Post

Jake From State Farm Net Worth: How Rich Is He? A Closer Look at the Growth of a Modern Financial Success Story

The Hidden Detail You Missed in Shrek—Black Suit, The Unseen Detail, and Its Disturbing Legacy

Is Jasmine Crockett Wedding Status Unveiled? Official Update Falls into the Spotlight

Sidney Barney: The Rise Of A Modern Icon

