What Is Equivalence Point in Titration? The Science Behind Chemical Reaction Completion
What Is Equivalence Point in Titration? The Science Behind Chemical Reaction Completion
The equivalence point in titration marks the precise moment when the reactants—analyte and titrant—have combined in stoichiometric proportions, defining the completion of a chemical reaction. Far more than a symbolic milestone, this pivotal point determines the accuracy and reliability of quantitative analysis in fields ranging from industrial chemistry to clinical diagnostics. Understanding equivalence—when moles of titrant exactly match those of analyte—is fundamental to interpreting titration curves, optimizing buffer systems, and ensuring measurement precision.
As analytical chemist Dr. Elena Torres notes, “The equivalence point is not just a number; it’s the moment chemistry reveals its balance—where every molecule counts with unwavering certainty.” Every titration hinges on a well-defined equivalence point, a concept central to acid-base, redox, and complexometric titrations. At this juncture, the solution’s composition shifts decisively, triggering a measurable change—whether a color shift, pH jump, or conductance spike—that signals the reaction’s end.
“Without defining this point,” explains Dr. James Lin, senior laboratory scientist, “we cannot accurately quantify unknown concentrations or determine if endpoint indicators are working. It’s the bridge between theory and real-world precision.”
Defining Equivalence: Stoichiometry Meets Measurement
At its core, the equivalence point is derived from stoichiometric proportions.In a simple acid-base titration of hydrochloric acid (HCl) with sodium hydroxide (NaOH), the reaction H⁺ + OH⁻ → H₂O proceeds with a 1:1 molar ratio. When exactly equal moles of H⁺ have reacted with OH⁻, equivalence is reached: \[ n_{\text{HCl}} = n_{\text{NaOH}} \] This equality allows direct calculation of the analyte concentration using known titrant volume and molarity. The equivalent point differs slightly from the pH equivalence point—where pH reflects neutralization—often occurring in a pH range influenced by strong acid–base compatibility, such as pH ~7 for monoprotic acids.
Quantifying equivalence depends on careful monitoring of reactant concentrations and reactive stoichiometry. Indicators or automated probes detect this transition by signaling when the solution’s chemistry fundamentally shifts. Sophisticated systems employ potentiometric or spectrophotometric detection, enabling precise equivalence moment capture even in complex mixtures.
“The equivalence point is the reaction’s truth,” says Dr. Lin. “It confirms not just presence of reactants, but their exact, complete consumption.”
Equivalence in Diverse Titration Methods
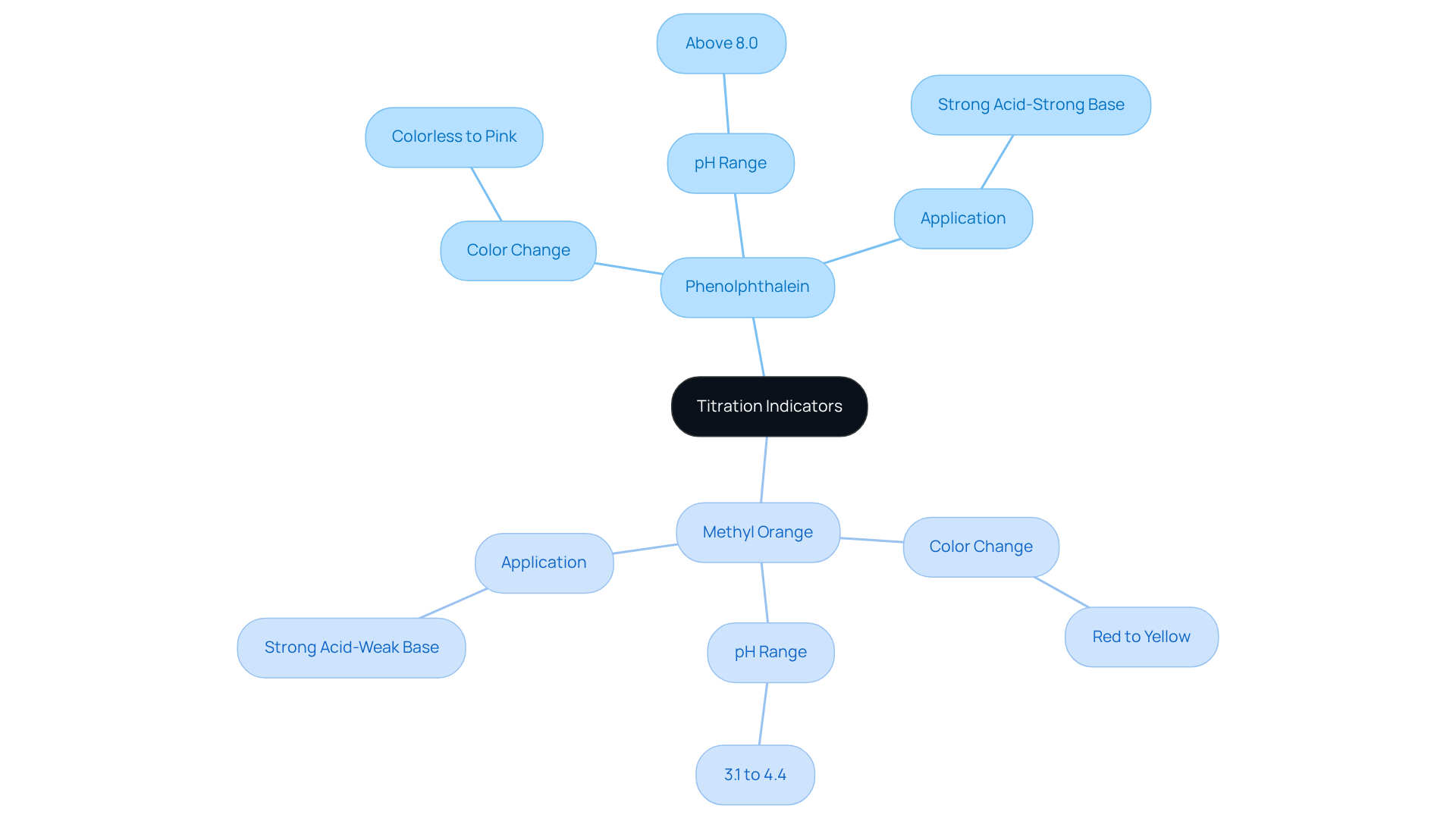
Not all titrations follow the same pathway—equivalence manifests differently across analytical methods, each demanding tailored detection strategies.In acid-base titrations, the equivalence point occurs as the pH crosses a sharp inflection, signaling complete proton transfer. In redox titrations, such as potassium permanganate (KMnO₄) oxidizing ferrous iron, equivalence corresponds to complete electron transfer, where all reducing species are converted to oxidized forms. Complexometric titrations using EDTA detect metal ion binding via color changes at calcium hardness equivalence, enabling precise quantification in water and industrial fluids.
“Each method requires calibration to its unique equivalence signature,” notes Dr. Torres. “For instance, a redox titration might use a rising permanganent color at equivalence, while a back-titration might measure unreacted excess titrant to infer analyte moles.
The principle — stoichiometric completeness — remains constant, but detection evolves with chemistry.”
Deriving Concentrations: From Equivalence to Analyte Determination
The equivalence point is indispensable for converting observed chemical changes into quantitative data. Once equivalence is identified—via pH meter, refractometer, or fading indicator—the reaction’s stoichiometry allows calculation of unknown analyte amounts. Using the formula: \[ M_{\text{analyte}} \times V_{\text{analyte}} = M_{\text{titrant}} \times V_{\text{equivalence}} \] scientific measurements transform into accurate concentration values.This rigor underpins applications from pharmaceutical quality control to environmental pollutant monitoring. For example, in a typical acid-base analysis, measuring 22.4 mL of 0.1 M NaOH required to neutralize a HCl sample hinges on identifying 22.4 mL as equivalence. The moles of NaOH then reveal the moles—and thus mass—of the original acid.
Such precision ensures goods meet regulatory standards and scientific claims are validated.
Challenges and Precision in Equivalence Detection
Despite its centrality, detecting equivalence is not without challenge. Indicator-based titrations risk subjective misjudgment, especially near pH transitions still influenced by weak acid/base dissociation.Automated titrators resolve this by tracking continuous pH or conductivity curves, identifying equivalence through inflection points or algorithmic analysis. These systems detect subtle shape changes imperceptible to the human eye, improving repeatability and reducing error. Modern advancements include conductometric and coulometric titrations, where equivalence is confirmed by abrupt shifts in ionic current or charge delivery—eliminating reliance on visual indicators.
“These techniques capture equivalence with superior precision,” says Dr. Lin. “They reduce ambiguity in complex matrices and enable real-time monitoring, critical for industrial processes and high-stakes research.”
The Indispensable Role of Equivalence in Modern Analysis
Understanding the equivalence point transforms titration from a simple lab technique into a powerful analytical discipline.It embodies the marriage of theoretical principles—stoichiometry and equilibrium—with practical measurement, enabling scientists to quantify substances with remarkable accuracy. “The equivalence point is where chemistry meets coincidence,” concludes Dr. Torres.
“It’s not just a moment to stop; it’s the moment that makes precise analysis possible.” From educational labs to global manufacturing, equivalence point determination remains foundational. Mastery of this concept ensures reliable data crucial to innovation, safety, and scientific integrity. As analytical demands grow more sophisticated, the equivalence point stands as a timeless benchmark—anchoring progress in chemistry’s relentless pursuit of precision.


Related Post

Chris Farley’s Tragic Demise: The Fatal Cost of a Legacy Shortened

Unlocking the Gate: Mastering the Requirements for Nin Registration in Today’s Digital Landscape
Nvidia Battery Boost: Should You Leave It Enabled for Better Performance and Endurance?

Travis Scott Bandanas: The Little Silk That Became a Cultural Philanthropy

