What Is Ionization Enthalpy? The Key to Understanding Atomic Energy Dynamics
What Is Ionization Enthalpy? The Key to Understanding Atomic Energy Dynamics
Ionization enthalpy—the energy required to remove an electron from a gaseous atom—is a fundamental metric that underpins much of modern chemistry and physics. It quantifies the stability of an atom’s electron configuration and reveals how readily atoms engage in chemical bonding or energy exchange. More than a mere thermodynamic value, ionization enthalpy serves as a predictive tool in fields ranging from materials science to astrophysics, shaping our understanding of element reactivity and atomic behavior.
At its core, ionization enthalpy reflects the enthalpic penalty of ejecting a single electron from a neutral atom in its ground state. This process, known as first ionization, captures the intrinsic strength of electron-nucleus binding. The higher the ionization energy, the more tightly electrons are held—making the atom less reactive in electron-donation scenarios.
For example, noble gases like argon exhibit exceptionally high ionization enthalpies, exceeding 1000 kJ/mol, due to their complete, stable electron shells, while alkali metals such as cesium have remarkably low values—around 375 kJ/mol—reflecting their ease of electron loss.
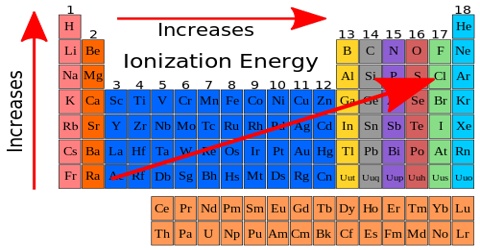
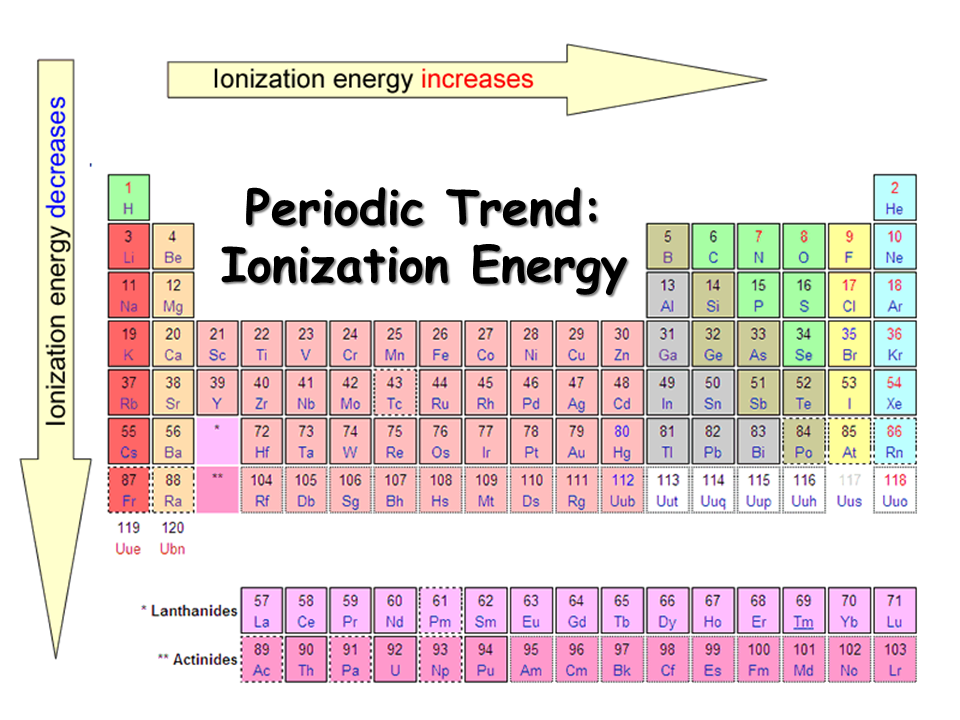
Understanding ionization enthalpy demands attention to periodic trends. Across a period, ionization energy generally increases: electrons occupy the same shell but face greater effective nuclear charge, tightening their grip on the nucleus. In contrast, atomic radius increases down a group, reducing the attractive force on outermost electrons and thus lowering ionization enthalpy.
However, exceptions exist—such as the dip in ionization energy between nitrogen and oxygen—driven by electron-electron repulsion in paired orbitals. These nuances reveal how subtle quantum effects govern macroscopic chemical behavior.
Measuring ionization enthalpy typically involves spectroscopic techniques or Calorimetry, both requiring precise control over experimental conditions. Modern approaches, like photoelectron spectroscopy, enable direct observation of electron binding energies with high resolution, while computational chemistry leverages quantum mechanical models to simulate ionization profiles.
These methods provide not just raw numbers but insight into valence electron dynamics, orbital overlap, and excitation impacts.
Real-world implications of ionization enthalpy span multiple scientific domains. In materials engineering, it guides the design of semiconductors and catalysts by identifying elements with optimal electron transport properties. For instance, silicon’s intermediate ionization energy makes it a cornerstone of modern electronics.
In astrophysics, spectral lines from ionized atoms inform analysis of stellar composition, where extreme temperatures result in readily ionized species whose energy thresholds reveal ionization states of interstellar matter.
Perhaps most compelling is ionization enthalpy’s role in unraveling biological electron transfer systems. Enzymes and pigments like cytochromes rely on precise electron removal and transfer, energies dictated by constituent atoms’ ionization thresholds. “The ionization enthalpy essentially defines the ‘activation hurdle’ for an electron’s escape,” explains a biophysicist specializing in photosynthesis.
“Without this energy parameter, predicting reaction kinetics across biomolecules would be guesswork.”
- Periodic Trends Deepen Predictive Power: Ionization energy increases predictably across periods due to rising effective nuclear charge, yet deviations—like the drop from Be to B—highlight electron configuration effects.
- Exceptions Underscore Complexity: Electron pairing in orbitals creates energy anomalies; for example, the lower ionization energy of oxygen compared to nitrogen counters algebraic predictions.
- Interdisciplinary Applications: From nanomaterials to stellar composition, ionization enthalpy bridges atomic-scale physics and large-scale natural phenomena.
- Experimental and Computational Synergy: Both physical measurement and theoretical modeling are vital, ensuring accuracy and expanding insight into elusive excited states.


Related Post

Cricket Wireless 5G Home Internet: Your Complete Guide to Ultra-Fast, Reliable Wireless Connectivity
Minibattles GitHub: how open-source innovation is splitting Battles.io into competitive micro-wars

Unveiling The Early Years Pictures Of Young Nicki Minaj When She Was Little
John Karl Fetterman’s Faith: Roots, Values, and Moral Compass in Public Service

