What Transforms the Cell: Unveiling the Vital Events of Prophase
What Transforms the Cell: Unveiling the Vital Events of Prophase
At the heart of cellular division lies prophase, a cornerstone stage where the delicate balance of genetics and structure shifts rapidly to prepare for division. This critical phase transforms a quiet, undivided cell into one poised to split into two genetically identical daughter cells. Through a tightly choreographed sequence of molecular events—chromosome condensation, nuclear envelope breakdown, spindle formation, and centriole movement—prophase ensures accuracy in genetic inheritance, safeguarding life at the most fundamental level.
Far more than a mechanical pause, prophase embodies a microscopic showdown between chaos and control, where precision prevents errors that could lead to disease or developmental disorders.
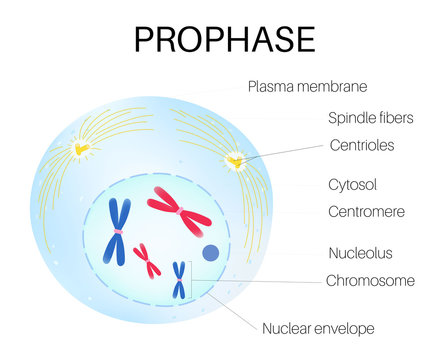
During prophase, the chromosome condensation process begins with dramatic structural changes. Chromatin, previously dispersed in a loose, transcription-competent state, begins compacting into distinct, rod-shaped chromosomes visible under light microscopy.
This transformation is driven by specialized proteins such as condensins and cohesins, which squeeze DNA into supercoiled, dense units, preventing tangles and facilitating equitable segregation later. As chromosomes condense, individual chromosomes become uniquely identifiable by their centromeres and arm ratios, setting the stage for their accurate alignment during metaphase. According to molecular biologist Dr.
Elena Vasquez, “Chromosome condensation is not merely a size change—it’s a transformation into a physically stable, division-ready form essential for error-free partitioning.”
Complementing chromosome packaging, the nuclear envelope undergoes a critical dismantling. Composed of a double lipid bilayer supported by intermediate filament proteins, the nuclear envelope partially breaks down during prophase. This process, mediated by phosphorylation of nuclear lamins and disassembly of nucleoporins, creates a permeable structure that allows motor proteins and spindle components access to the chromosomes.
This selective breakdown is carefully regulated—partial disintegration ensures that the nuclear import machinery does not prematurely restrict chromosome movement, yet permits essential contributions from the cytoskeleton. As the envelope fractures, nucleoli disappear, their dissolution reflecting the cell’s transition from an environment focused on transcription to one focused on division.
A defining feature of prophase is the assembly of the mitotic spindle—a dynamic network of microtubules orchestrated from centrosomes that have migrated to opposite poles of the cell. Tinese centrosomes, rich in γ-tubulin ring complexes, nucleate microtubules that rapidly grow and stabilize into spindle fibers.
These spindle components interact with chromosome kinetochores—protein structures draped across each sister chromatid pair—ensuring mechanical linkage. “Microtubule-kinetochore attachments are the molecular glue of mitosis,” explains cell biologist Dr. James Kim.
“They enable precise pulling forces necessary for chromosome alignment, forming the engine room of cell division.” Spindle dynamics—centrosome separation, microtubule nucleation, and motor-driven searching—create a dynamic balance tunable by checkpoints, particularly the spindle assembly checkpoint (SAC), which delays anaphase until all chromosomes are correctly secured.
Beyond structural foundations, prophase marks the activation of key regulatory systems that monitor and direct division. Cyclin-dependent kinases (CDKs), especially CDK1 in complex with cyclin B, phosphorylate hundreds of target proteins, unlocking structural changes while inhibiting premature processes like cytokinesis. The SAC, centered on proteins such as Mad2 and BubR1, serves as a safeguard: if even one kinetochore remains unattached, the checkpoint halts progression, giving time for correction.
This surveillance prevents missegregation—a leading cause of aneuploidy in cancer and developmental conditions. “Prophase is nature’s precision control center,” notes Dr. Vasquez.
“Without it, the genome’s integrity would be at constant risk, undermining organismal health.”
Prophase holds profound biological significance beyond basic reproduction. Errors in its progression frequently manifest in human disease—nondisjunction during meiosis causes chromosomal disorders like Down syndrome, while defective mitotic spindling contributes to tumor progression. Advances in live-cell imaging and super-resolution microscopy now reveal prophase dynamics in unprecedented detail, shedding light on why some cells divide flawlessly while others develop fatal flaws.
Researchers are increasingly focusing on prophase as a therapeutic target; drugs that disrupt spindle formation or kinase signaling show promise in oncology. Still, mastering prophase’s intricacies demands continuous exploration—each checkpoint, microtubule, and condensin interaction representing a vital cog in life’s most fundamental process.
In essence, prophase is far more than the beginning of mitosis—it is a meticulously governed transformation where biology’s precision meets mechanical complexity. Through condensed chromosomes, dismantling envelopes, spindle architecture, and vigilant checkpoints, cells prepare for division with remarkable accuracy.
Understanding what unfolds in this brief, pivotal phase deepens our grasp of life’s continuity and equips science to address some of medicine’s most enduring challenges.



Related Post

Unveiling The Mystery: Where Did Dove Cameron Get Her Last Name?

Moner Kotha Mone Thakuk: The Lyrical Genius Behind Bangladesh’s Soulful Anthem

The Ice Cream Hair Trend: Where Flavor Meets Hair Color Revolution

A Detailed Look Into Diane Wildenstein’s Life, Career, and Enduring Legacy—Together with Dine Dughter, Jocelyn Closer, and Shared Mentorship

